

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin receptor type A (RAT) | BDBM50003666 (3-[2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity against cholecystokinin type A receptor from rat pancreas binding assay | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003669 (Ac-Tyr(SO3H)-Met-Gly-Trp-Met-R-Dtc-Phe-NH2 | CHEMB...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Concentration inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type A receptor of rat pancreatic membranes. | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity against cholecystokinin type A receptor of rat pancreas | J Med Chem 37: 3639-54 (1994) BindingDB Entry DOI: 10.7270/Q2SB46ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type A receptor in rat pancreas. | Bioorg Med Chem Lett 3: 871-874 (1993) Article DOI: 10.1016/S0960-894X(00)80683-6 BindingDB Entry DOI: 10.7270/Q2513Z41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]- CCK-33 to rat pancreas | J Med Chem 33: 591-5 (1990) BindingDB Entry DOI: 10.7270/Q29K4BTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Half maximal inhibition of specific binding of [125I]-Bolton-Hunter CCK-8 to Cholecystokinin type A receptor in the rat pancreas | J Med Chem 43: 3505-17 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9XBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of Cholecystokinin type A receptor in rat pancreatic tissue | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Research Laboratories Curated by ChEMBL | Assay Description Half-maximal inhibition of [125I]CCK-33 binding to cholecystokinin A receptor from rat pancreatic tissue | J Med Chem 31: 2235-46 (1989) BindingDB Entry DOI: 10.7270/Q2PG1S9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type A receptor in rat pancreas | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Cholecystokinin-8 (125I-CCK-8) binding to Cholecystokinin type A receptor of rat pancreatic membranes | Bioorg Med Chem Lett 7: 169-174 (1997) Article DOI: 10.1016/S0960-894X(96)00609-9 BindingDB Entry DOI: 10.7270/Q27M07ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125 I] CCK-8 from Cholecystokinin type A receptor of rat pancreas | J Med Chem 32: 13-6 (1989) BindingDB Entry DOI: 10.7270/Q2MC90MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50106593 (CHEMBL135602 | [1-(2-Benzyl-3-oxo-1-thioxo-octahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]pCCK-8 binding to cholecystokinin type A receptor of rat pancreas | J Med Chem 44: 4196-206 (2001) BindingDB Entry DOI: 10.7270/Q2TD9Z23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50170765 (CHEMBL363916 | [(S)-1-((4aS,5R)-2-Benzyl-1-mercapt...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of 71 pM [125I]BH-(Thr,Nle)CCK-9 binding to rat cholecystokinin 1 receptor | J Med Chem 48: 4842-50 (2005) Article DOI: 10.1021/jm0501127 BindingDB Entry DOI: 10.7270/Q2WQ03BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50170765 (CHEMBL363916 | [(S)-1-((4aS,5R)-2-Benzyl-1-mercapt...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of 71 pM [125I]BH-(Thr,Nle)CCK-9 binding to rat cholecystokinin 1 receptor | J Med Chem 48: 4842-50 (2005) Article DOI: 10.1021/jm0501127 BindingDB Entry DOI: 10.7270/Q2WQ03BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre Curated by ChEMBL | Assay Description Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type A receptor in the rat pancreas. | J Med Chem 36: 552-65 (1993) BindingDB Entry DOI: 10.7270/Q2JM2B88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type A receptor of rat pancreas | Bioorg Med Chem Lett 3: 889-894 (1993) Article DOI: 10.1016/S0960-894X(00)80687-3 BindingDB Entry DOI: 10.7270/Q28G8M63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit Curated by ChEMBL | Assay Description Half-maximal inhibition of specific binding of [125I]bolton hunter CCK-8 to rat pancreas cholecystokinin type A receptor | J Med Chem 34: 404-14 (1991) BindingDB Entry DOI: 10.7270/Q2RJ4K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit Curated by ChEMBL | Assay Description Half-maximal inhibition of specific binding of [125I]bolton hunter CCK-8 to rat pancreas cholecystokinin type A receptor | J Med Chem 34: 404-14 (1991) BindingDB Entry DOI: 10.7270/Q2RJ4K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre Curated by ChEMBL | Assay Description Evaluated for inhibition of cholecystokinin type A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas | J Med Chem 35: 2573-81 (1992) BindingDB Entry DOI: 10.7270/Q2TT4RJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50033600 (Asp-Tyr(SO3H)-Met-Gly-Trp-Met-Asp-Phe-NH2 | CHEMBL...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre Curated by ChEMBL | Assay Description Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type A receptor in the rat pancreas. | J Med Chem 36: 552-65 (1993) BindingDB Entry DOI: 10.7270/Q2JM2B88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre Curated by ChEMBL | Assay Description Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type A receptor in the rat pancreas. | J Med Chem 36: 552-65 (1993) BindingDB Entry DOI: 10.7270/Q2JM2B88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50281617 (Asp Tyr (OSO3H) Met Gly Trp Met Asp Phe | CHEMBL26...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition of specific binding of [125I]-Bolton Hunter CCK-8 to Cholecystokinin type A receptor in the rat pancreas | Bioorg Med Chem Lett 3: 667-670 (1993) Article DOI: 10.1016/S0960-894X(01)81250-6 BindingDB Entry DOI: 10.7270/Q2K937G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50023142 (1H-Indole-2-carboxylic acid [6-(2-fluoro-phenyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-CCK from Cholecystokinin receptor of rat pancreas | J Med Chem 31: 176-81 (1988) BindingDB Entry DOI: 10.7270/Q2ZS2VH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50026690 (2-[2-(2-{2-[2-{2-[2-(2-Amino-3-phenyl-propionylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of the binding of [125I]-(Nle11)-HG-13 to Histamine H2 receptor | J Med Chem 27: 1597-601 (1985) BindingDB Entry DOI: 10.7270/Q29P30PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50011545 (CHEMBL430906 | Desamino-Tyr(SO3H)-Met-Gly-Trp-Met-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type A receptor was determined in fresh rat pancreatic tissue membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50456295 (CHEMBL2111920) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]CCK-8 to cholecystokinin type A receptor in rat pancreatic tissue | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type A receptor of rat pancreas | Bioorg Med Chem Lett 3: 889-894 (1993) Article DOI: 10.1016/S0960-894X(00)80687-3 BindingDB Entry DOI: 10.7270/Q28G8M63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50367648 (CHEMBL1907661) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Research Laboratories Curated by ChEMBL | Assay Description Half-maximal inhibition of [125I]CCK-8 binding to cholecystokinin receptor from rat pancreatic tissue | J Med Chem 31: 2235-46 (1989) BindingDB Entry DOI: 10.7270/Q2PG1S9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50281730 (4-Chloro-N-((S)-1-methyl-2-oxo-5-phenyl-2,3-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type A receptor in rat pancreas | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50367623 (CHEMBL1907852) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125 I] CCK-8 from Cholecystokinin type A receptor of rat pancreas | J Med Chem 32: 13-6 (1989) BindingDB Entry DOI: 10.7270/Q2MC90MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50280834 (1-((S)-5-Cyclohexyl-1-methyl-2-oxo-2,3-dihydro-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.455 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro test for inhibition of [125I]-CCK binding to Cholecystokinin type A receptor from rat pancreatic tissues was determined | Bioorg Med Chem Lett 3: 1919-1924 (1993) Article DOI: 10.1016/S0960-894X(01)80987-2 BindingDB Entry DOI: 10.7270/Q23J3CWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50154434 ((S)-2-((4S,5S)-2-Benzyl-4-methyl-1,3-dioxo-octahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]-pCCK-8 binding to Cholecystokinin type A receptor of rat pancreas homogenates | J Med Chem 47: 5318-29 (2004) Article DOI: 10.1021/jm0498755 BindingDB Entry DOI: 10.7270/Q2VX0H8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rotta Research Laboratorium Curated by ChEMBL | Assay Description Concentration required to inhibit by 50% specific binding of [125I](BH)-CCK-8 to cholecystokinin type A receptor in rat pancreatic acini | J Med Chem 35: 28-38 (1992) BindingDB Entry DOI: 10.7270/Q2XS5W11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50170764 (CHEMBL366344 | [(S)-2-(1H-Indol-3-yl)-1-((4aS,5R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of 71 pM [125I]BH-(Thr,Nle)CCK-9 binding to rat cholecystokinin 1 receptor | J Med Chem 48: 4842-50 (2005) Article DOI: 10.1021/jm0501127 BindingDB Entry DOI: 10.7270/Q2WQ03BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50170764 (CHEMBL366344 | [(S)-2-(1H-Indol-3-yl)-1-((4aS,5R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of 71 pM [125I]BH-(Thr,Nle)CCK-9 binding to rat cholecystokinin 1 receptor | J Med Chem 48: 4842-50 (2005) Article DOI: 10.1021/jm0501127 BindingDB Entry DOI: 10.7270/Q2WQ03BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003200 (CHEMBL267849 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of specific [3H]-propanoyl-CCK-8 binding to cholecystokinin type A receptor in rat pancreatic membran... | J Med Chem 35: 3774-83 (1992) BindingDB Entry DOI: 10.7270/Q2CC0ZMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003670 (3-{2-[2-(2-{2-[2-Acetylamino-3-(4-sulfo-phenyl)-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Concentration inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type A receptor of rat pancreatic membranes. | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50367639 (CHEMBL1907855) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.603 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity against cholecystokinin type A receptor of rat pancreas | J Med Chem 37: 3639-54 (1994) BindingDB Entry DOI: 10.7270/Q2SB46ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50290400 (1H-Indole-2-carboxylic acid [(S)-1-(2-fluoro-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Cholecystokinin-8 (125I-CCK-8) binding to Cholecystokinin type A receptor of rat pancreatic membranes | Bioorg Med Chem Lett 7: 169-174 (1997) Article DOI: 10.1016/S0960-894X(96)00609-9 BindingDB Entry DOI: 10.7270/Q27M07ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding of [3H]-propionyl-CCK-8 to Cholecystokinin type A receptor from rat pancreas | Bioorg Med Chem Lett 6: 967-972 (1996) Article DOI: 10.1016/0960-894X(96)00160-6 BindingDB Entry DOI: 10.7270/Q2736QWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Insituto de Qu�mica M�dica (CSIC), Juan de la Cierva 3, E-28006 Madrid, Spain. Curated by ChEMBL | Assay Description Binding affinity by competitive inhibition of the radioligand [3H]pCCK-8 at Cholecystokinin type A receptor from rat pancreas | J Med Chem 42: 4659-68 (1999) BindingDB Entry DOI: 10.7270/Q24T6K2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description In vitro inhibition of [3H]propionyl-CCK-8 binding to rat pancreatic membranes at Cholecystokinin type A receptor. | J Med Chem 43: 3770-7 (2000) BindingDB Entry DOI: 10.7270/Q21J9BG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50019231 (CHEMBL73946 | N-[5-(2-Fluoro-phenyl)-1-methyl-2-ox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Research Laboratories Curated by ChEMBL | Assay Description Half-maximal inhibition of [125I]CCK-33 binding to cholecystokinin A receptor from rat pancreatic tissue | J Med Chem 31: 2235-46 (1989) BindingDB Entry DOI: 10.7270/Q2PG1S9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50019231 (CHEMBL73946 | N-[5-(2-Fluoro-phenyl)-1-methyl-2-ox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.759 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity against cholecystokinin type A receptor of rat pancreas | J Med Chem 37: 3639-54 (1994) BindingDB Entry DOI: 10.7270/Q2SB46ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50019181 ((S)-4-Bromo-N-[5-(2-fluoro-phenyl)-1-methyl-2-oxo-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity against cholecystokinin type A receptor of rat pancreas | J Med Chem 37: 3639-54 (1994) BindingDB Entry DOI: 10.7270/Q2SB46ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50019259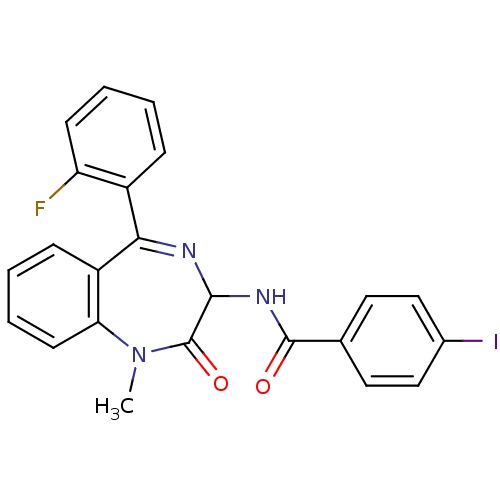 ((S)-N-[5-(2-Fluoro-phenyl)-1-methyl-2-oxo-2,3-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity against cholecystokinin type A receptor of rat pancreas | J Med Chem 37: 3639-54 (1994) BindingDB Entry DOI: 10.7270/Q2SB46ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity against cholecystokinin type A receptor of rat pancreas | J Med Chem 37: 3639-54 (1994) BindingDB Entry DOI: 10.7270/Q2SB46ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50452486 (CHEMBL39263 | Rac-Devazepide) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Research Laboratories Curated by ChEMBL | Assay Description Half-maximal inhibition of [125I]CCK-33 binding to cholecystokinin A receptor from rat pancreatic tissue | J Med Chem 31: 2235-46 (1989) BindingDB Entry DOI: 10.7270/Q2PG1S9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50367626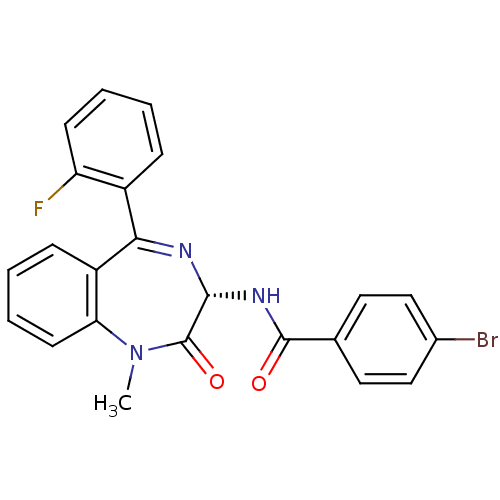 (CHEMBL1907850) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Research Laboratories Curated by ChEMBL | Assay Description Half-maximal inhibition of [125I]CCK-33 binding to cholecystokinin A receptor from rat pancreatic tissue | J Med Chem 31: 2235-46 (1989) BindingDB Entry DOI: 10.7270/Q2PG1S9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Research Laboratories Curated by ChEMBL | Assay Description Half-maximal inhibition of binding of [125I]CCK-8 to Cholecystokinin receptor in rat pancreatic tissue | J Med Chem 32: 1681-5 (1989) BindingDB Entry DOI: 10.7270/Q21838QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1380 total ) | Next | Last >> |