

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50581207 (CHEMBL5074216) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at muscarinic M3 receptor in rat trachea assessed as inhibition of carbachol-induced contraction | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50581207 (CHEMBL5074216) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at muscarinic M3 receptor in rat trachea assessed as inhibition of carbachol-induced contraction | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50581203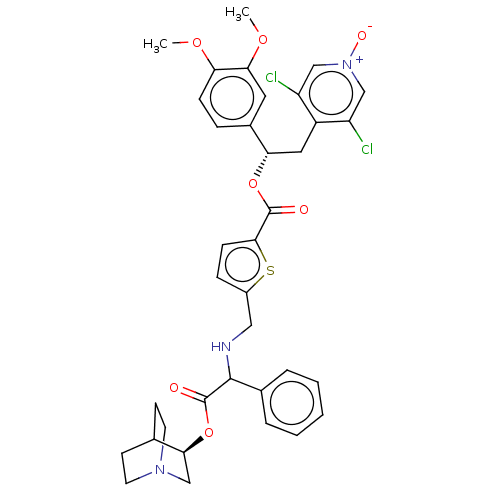 (CHEMBL5074599) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at muscarinic M3 receptor in rat trachea assessed as inhibition of carbachol-induced contraction | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50581203 (CHEMBL5074599) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at muscarinic M3 receptor in rat trachea assessed as inhibition of carbachol-induced contraction | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50581206 (CHEMBL5076886) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at muscarinic M3 receptor in rat trachea assessed as inhibition of carbachol-induced contraction | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM325606 (US9636336, Example 87c) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at muscarinic M3 receptor in rat trachea assessed as inhibition of carbachol-induced contraction | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM325637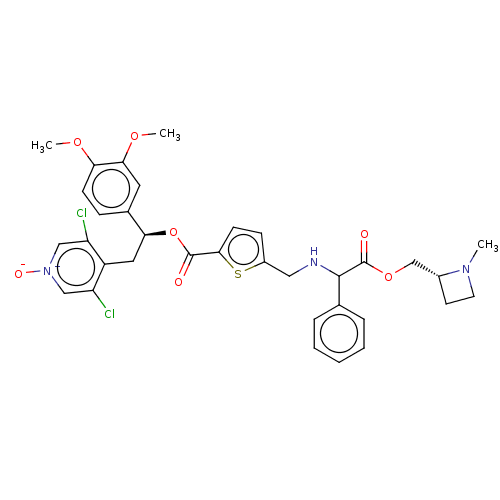 (US9636336, Example 28 | US9636336, Example 94 | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at muscarinic M3 receptor in rat trachea assessed as inhibition of carbachol-induced contraction | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM325637 (US9636336, Example 28 | US9636336, Example 94 | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at muscarinic M3 receptor in rat trachea assessed as inhibition of carbachol-induced contraction | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM325606 (US9636336, Example 87c) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at muscarinic M3 receptor in rat trachea assessed as inhibition of carbachol-induced contraction | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM325621 (US9636336, Example 11 | US9636336, Example 65 | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at muscarinic M3 receptor in rat trachea assessed as inhibition of carbachol-induced contraction | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM325621 (US9636336, Example 11 | US9636336, Example 65 | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at muscarinic M3 receptor in rat trachea assessed as inhibition of carbachol-induced contraction | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM325605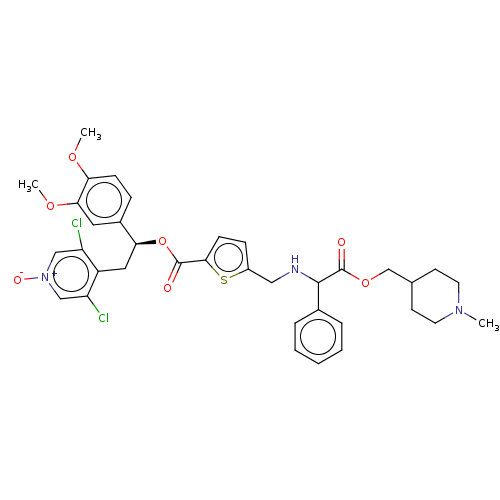 (US9636336, Example 23 | US9636336, Example 86 | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at muscarinic M3 receptor in rat trachea assessed as inhibition of carbachol-induced contraction | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM325605 (US9636336, Example 23 | US9636336, Example 86 | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at muscarinic M3 receptor in rat trachea assessed as inhibition of carbachol-induced contraction | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018304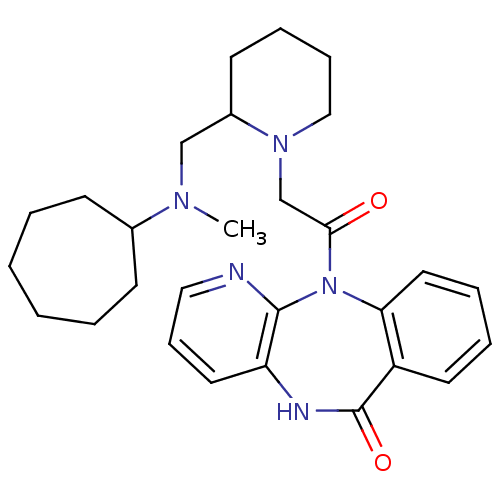 (11-(2-{2-[(Cycloheptyl-methyl-amino)-methyl]-piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Binding affinity for glandular muscarinic acetylcholine receptor M3 in rat assayed using 0.3 nM [3H]-N-methylscopolamine as radioligand | J Med Chem 32: 1718-24 (1989) BindingDB Entry DOI: 10.7270/Q2XG9Q4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018304 (11-(2-{2-[(Cycloheptyl-methyl-amino)-methyl]-piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of rat submandibular muscarinic (M3) receptor isolated from tissue homogenates | J Med Chem 37: 3775-88 (1994) BindingDB Entry DOI: 10.7270/Q2QC02JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50581208 (CHEMBL5076535) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at muscarinic M3 receptor in rat trachea assessed as inhibition of carbachol-induced contraction | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50581208 (CHEMBL5076535) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79.4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at muscarinic M3 receptor in rat trachea assessed as inhibition of carbachol-induced contraction | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018314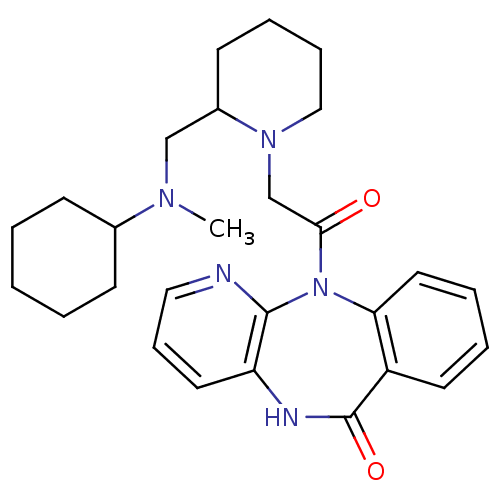 (11-(2-{2-[(Cyclohexyl-methyl-amino)-methyl]-piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of rat submandibular muscarinic (M3) receptor isolated from tissue homogenates | J Med Chem 37: 3775-88 (1994) BindingDB Entry DOI: 10.7270/Q2QC02JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018314 (11-(2-{2-[(Cyclohexyl-methyl-amino)-methyl]-piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Binding affinity for glandular muscarinic acetylcholine receptor M3 in rat assayed using 0.3 nM [3H]-N-methylscopolamine as radioligand | J Med Chem 32: 1718-24 (1989) BindingDB Entry DOI: 10.7270/Q2XG9Q4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018298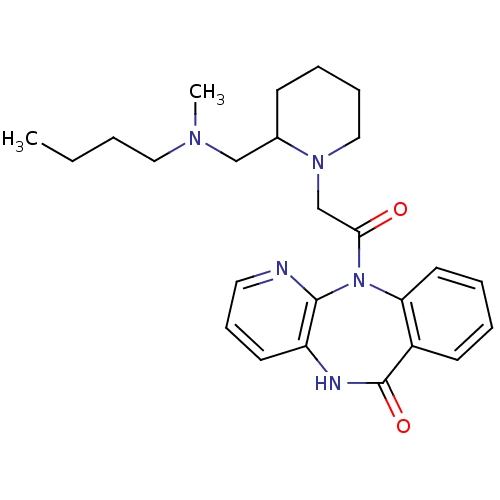 (11-(2-{2-[(Butyl-methyl-amino)-methyl]-piperidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Binding affinity for glandular muscarinic acetylcholine receptor M3 in rat assayed using 0.3 nM [3H]-N-methylscopolamine as radioligand | J Med Chem 32: 1718-24 (1989) BindingDB Entry DOI: 10.7270/Q2XG9Q4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018298 (11-(2-{2-[(Butyl-methyl-amino)-methyl]-piperidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of rat submandibular muscarinic (M3) receptor isolated from tissue homogenates | J Med Chem 37: 3775-88 (1994) BindingDB Entry DOI: 10.7270/Q2QC02JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018291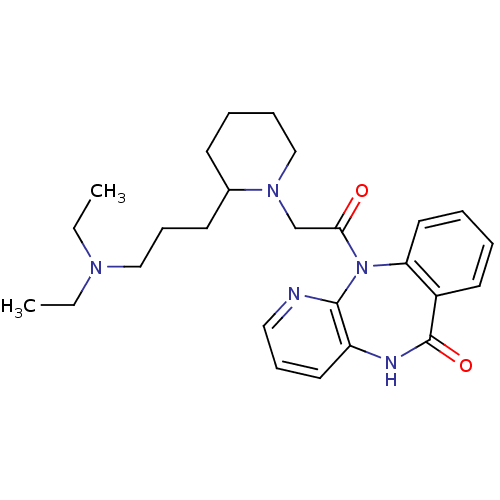 (11-{2-[2-(3-Diethylamino-propyl)-piperidin-1-yl]-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of rat submandibular muscarinic (M3) receptor isolated from tissue homogenates | J Med Chem 37: 3775-88 (1994) BindingDB Entry DOI: 10.7270/Q2QC02JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018291 (11-{2-[2-(3-Diethylamino-propyl)-piperidin-1-yl]-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Binding affinity for glandular muscarinic acetylcholine receptor M3 in rat assayed using 0.3 nM [3H]-N-methylscopolamine as radioligand | J Med Chem 32: 1718-24 (1989) BindingDB Entry DOI: 10.7270/Q2XG9Q4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018313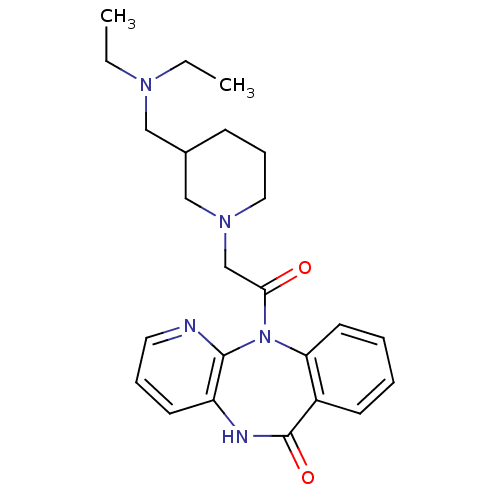 (11-[2-(3-Diethylaminomethyl-piperidin-1-yl)-acetyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of rat submandibular muscarinic (M3) receptor isolated from tissue homogenates | J Med Chem 37: 3775-88 (1994) BindingDB Entry DOI: 10.7270/Q2QC02JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018313 (11-[2-(3-Diethylaminomethyl-piperidin-1-yl)-acetyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Binding affinity for glandular muscarinic acetylcholine receptor M3 in rat assayed using 0.3 nM [3H]-N-methylscopolamine as radioligand | J Med Chem 32: 1718-24 (1989) BindingDB Entry DOI: 10.7270/Q2XG9Q4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM39341 (11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche - Università di Genova Curated by ChEMBL | Assay Description Displacement of [3H]-methylscopolamine binding to muscarinic M3 receptor in submaxillary salivary glands of rats. | Bioorg Med Chem Lett 9: 3031-4 (1999) BindingDB Entry DOI: 10.7270/Q2V40TD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50462906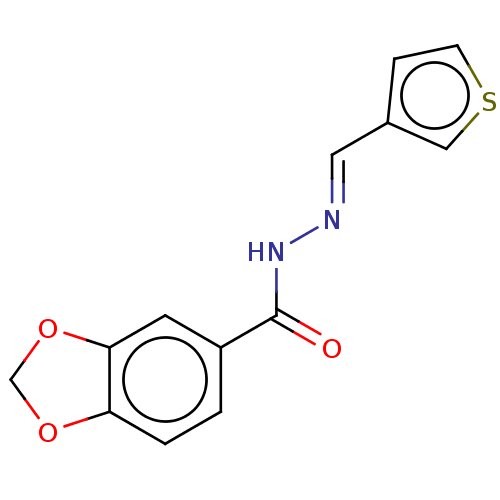 (CHEMBL3211778) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute for Science and Technology on Innovation on Neglected Diseases (INCT/IDN) Curated by ChEMBL | Assay Description Agonist activity at endothelial M3 receptor in Wistar-Kyoto rat thoracic aortic rings assessed as inhibition of phenylephrine-induced contraction | Bioorg Med Chem Lett 28: 2797-2806 (2018) Article DOI: 10.1016/j.bmcl.2018.07.015 BindingDB Entry DOI: 10.7270/Q2CR5X0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018300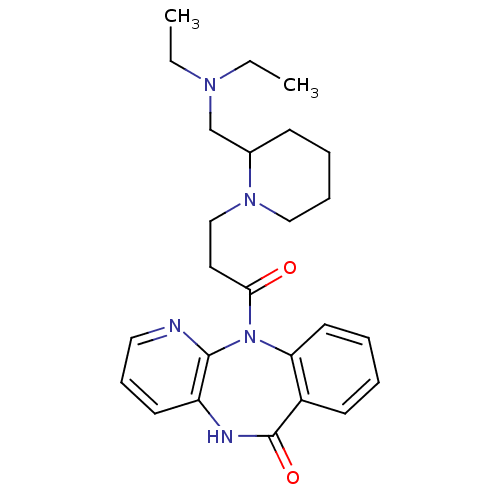 (11-[3-(2-Diethylaminomethyl-piperidin-1-yl)-propio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of rat submandibular muscarinic (M3) receptor isolated from tissue homogenates | J Med Chem 37: 3775-88 (1994) BindingDB Entry DOI: 10.7270/Q2QC02JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM39341 (11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Binding affinity for glandular muscarinic acetylcholine receptor M3 in rat assayed using 0.3 nM [3H]-N-methylscopolamine as radioligand | J Med Chem 32: 1718-24 (1989) BindingDB Entry DOI: 10.7270/Q2XG9Q4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018300 (11-[3-(2-Diethylaminomethyl-piperidin-1-yl)-propio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Binding affinity for glandular muscarinic acetylcholine receptor M3 in rat assayed using 0.3 nM [3H]-N-methylscopolamine as radioligand | J Med Chem 32: 1718-24 (1989) BindingDB Entry DOI: 10.7270/Q2XG9Q4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50082333 (11-[2-(Octahydro-quinolizin-1-ylmethylsulfanyl)-ac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche - Università di Genova Curated by ChEMBL | Assay Description Displacement of [3H]-methylscopolamine binding to muscarinic M3 receptor in submaxillary salivary glands of rats. | Bioorg Med Chem Lett 9: 3031-4 (1999) BindingDB Entry DOI: 10.7270/Q2V40TD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50082335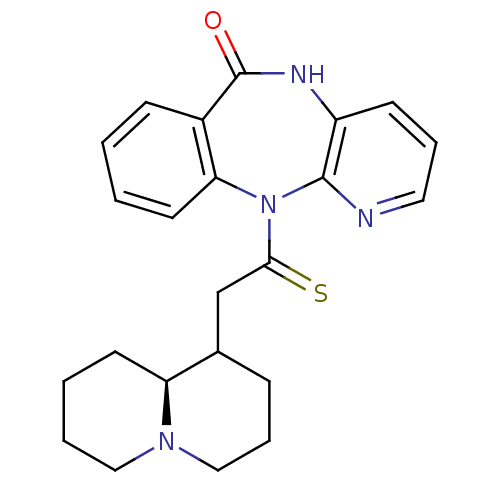 (11-((S)-2-Octahydro-quinolizin-1-yl-thioacetyl)-5,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche - Università di Genova Curated by ChEMBL | Assay Description Displacement of [3H]-methylscopolamine binding to muscarinic M3 receptor in submaxillary salivary glands of rats. | Bioorg Med Chem Lett 9: 3031-4 (1999) BindingDB Entry DOI: 10.7270/Q2V40TD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50082334 (11-{2-[(Octahydro-quinolizin-1-ylmethyl)-amino]-ac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche - Università di Genova Curated by ChEMBL | Assay Description Displacement of [3H]-methylscopolamine binding to muscarinic M3 receptor in submaxillary salivary glands of rats. | Bioorg Med Chem Lett 9: 3031-4 (1999) BindingDB Entry DOI: 10.7270/Q2V40TD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018307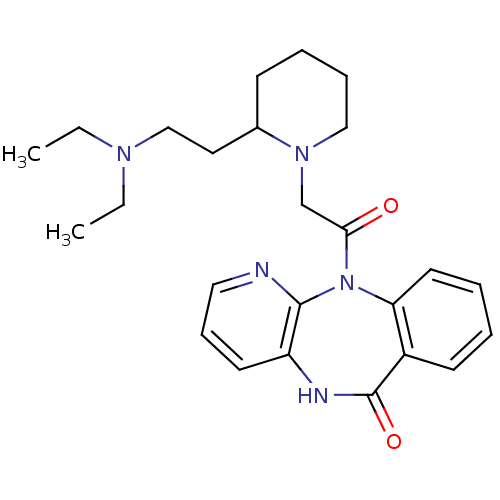 (11-{2-[2-(2-Diethylamino-ethyl)-piperidin-1-yl]-ac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Binding affinity for glandular muscarinic acetylcholine receptor M3 in rat assayed using 0.3 nM [3H]-N-methylscopolamine as radioligand | J Med Chem 32: 1718-24 (1989) BindingDB Entry DOI: 10.7270/Q2XG9Q4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018297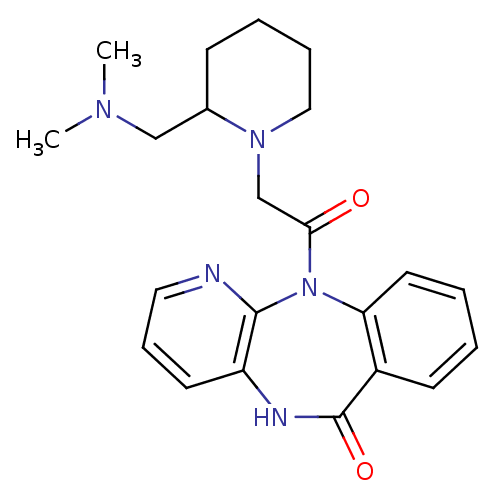 (11-[2-(2-Dimethylaminomethyl-piperidin-1-yl)-acety...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Binding affinity for glandular muscarinic acetylcholine receptor M3 in rat assayed using 0.3 nM [3H]-N-methylscopolamine as radioligand | J Med Chem 32: 1718-24 (1989) BindingDB Entry DOI: 10.7270/Q2XG9Q4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018292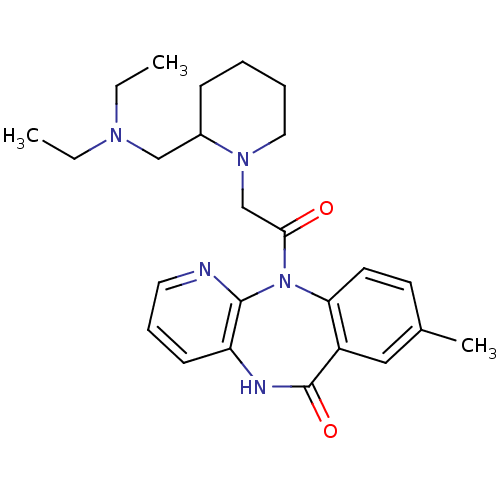 (11-[2-(2-Diethylaminomethyl-piperidin-1-yl)-acetyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Binding affinity for glandular muscarinic acetylcholine receptor M3 in rat assayed using 0.3 nM [3H]-N-methylscopolamine as radioligand | J Med Chem 32: 1718-24 (1989) BindingDB Entry DOI: 10.7270/Q2XG9Q4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50007674 ((+)-erythro 4-[2-(4-Benzyl-piperidin-1-yl)-1-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Compound was tested for the binding affinity against muscarinic acetylcholine receptor by using [3H]QNB as radioligand | J Med Chem 34: 3085-90 (1991) BindingDB Entry DOI: 10.7270/Q2FN155W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50034043 (1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor using [3H]quinuclidinyl benzilate | J Med Chem 38: 1119-31 (1995) BindingDB Entry DOI: 10.7270/Q2S75H08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018297 (11-[2-(2-Dimethylaminomethyl-piperidin-1-yl)-acety...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of rat submandibular muscarinic (M3) receptor isolated from tissue homogenates | J Med Chem 37: 3775-88 (1994) BindingDB Entry DOI: 10.7270/Q2QC02JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018292 (11-[2-(2-Diethylaminomethyl-piperidin-1-yl)-acetyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of rat submandibular muscarinic (M3) receptor isolated from tissue homogenates | J Med Chem 37: 3775-88 (1994) BindingDB Entry DOI: 10.7270/Q2QC02JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018307 (11-{2-[2-(2-Diethylamino-ethyl)-piperidin-1-yl]-ac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of rat submandibular muscarinic (M3) receptor isolated from tissue homogenates | J Med Chem 37: 3775-88 (1994) BindingDB Entry DOI: 10.7270/Q2QC02JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50082336 (CHEMBL322378 | N-(4-Benzoyl-2-methyl-phenyl)-2-(R)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche - Università di Genova Curated by ChEMBL | Assay Description Displacement of [3H]-methylscopolamine binding to muscarinic M3 receptor in submaxillary salivary glands of rats. | Bioorg Med Chem Lett 9: 3031-4 (1999) BindingDB Entry DOI: 10.7270/Q2V40TD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018293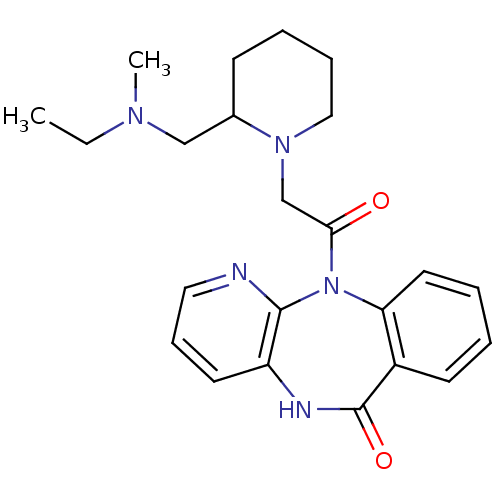 (11-(2-{2-[(Ethyl-methyl-amino)-methyl]-piperidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Binding affinity for glandular muscarinic acetylcholine receptor M3 in rat assayed using 0.3 nM [3H]-N-methylscopolamine as radioligand | J Med Chem 32: 1718-24 (1989) BindingDB Entry DOI: 10.7270/Q2XG9Q4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018311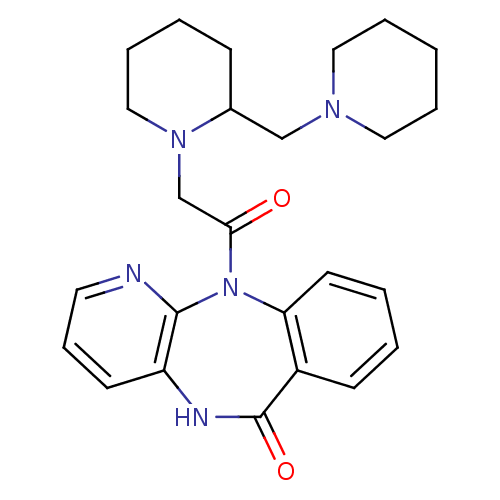 (11-[2-(2-Piperidin-1-ylmethyl-piperidin-1-yl)-acet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Binding affinity of compound for glandular Muscarinic acetylcholine receptor M3 in rat using 0.3 nM [3H]-N-methylscopolamine as radioligand | J Med Chem 32: 1718-24 (1989) BindingDB Entry DOI: 10.7270/Q2XG9Q4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018310 (11-[2-(2-Pyrrolidin-1-ylmethyl-piperidin-1-yl)-ace...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of rat submandibular muscarinic (M3) receptor isolated from tissue homogenates | J Med Chem 37: 3775-88 (1994) BindingDB Entry DOI: 10.7270/Q2QC02JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018302 (9-Chloro-11-[2-(2-diethylaminomethyl-piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Binding affinity for glandular muscarinic acetylcholine receptor M3 in rat assayed using 0.3 nM [3H]-N-methylscopolamine as radioligand | J Med Chem 32: 1718-24 (1989) BindingDB Entry DOI: 10.7270/Q2XG9Q4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018302 (9-Chloro-11-[2-(2-diethylaminomethyl-piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of rat submandibular muscarinic (M3) receptor isolated from tissue homogenates | J Med Chem 37: 3775-88 (1994) BindingDB Entry DOI: 10.7270/Q2QC02JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018311 (11-[2-(2-Piperidin-1-ylmethyl-piperidin-1-yl)-acet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of rat submandibular muscarinic (M3) receptor isolated from tissue homogenates | J Med Chem 37: 3775-88 (1994) BindingDB Entry DOI: 10.7270/Q2QC02JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018293 (11-(2-{2-[(Ethyl-methyl-amino)-methyl]-piperidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of rat submandibular muscarinic (M3) receptor isolated from tissue homogenates | J Med Chem 37: 3775-88 (1994) BindingDB Entry DOI: 10.7270/Q2QC02JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50018310 (11-[2-(2-Pyrrolidin-1-ylmethyl-piperidin-1-yl)-ace...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Binding affinity for glandular muscarinic acetylcholine receptor M3 in rat assayed using 0.3 nM [3H]-N-methylscopolamine as radioligand | J Med Chem 32: 1718-24 (1989) BindingDB Entry DOI: 10.7270/Q2XG9Q4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 109 total ) | Next | Last >> |