 Found 5 hits of ki for UniProtKB: P25102
Found 5 hits of ki for UniProtKB: P25102 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H2 receptor
(RAT) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Italia
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 352: 276-82 (1995)
Article DOI: 10.1007/bf00168557
BindingDB Entry DOI: 10.7270/Q2Q52N48 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(RAT) | BDBM50543288
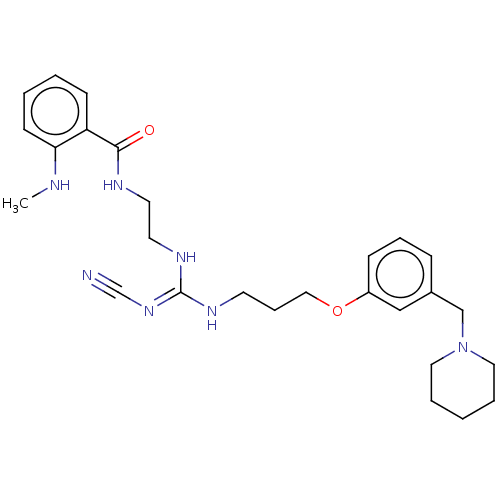
(CHEMBL4638742)Show SMILES CNc1ccccc1C(=O)NCCN\C(NCCCOc1cccc(CN2CCCCC2)c1)=N/C#N Show InChI InChI=1S/C27H37N7O2/c1-29-25-12-4-3-11-24(25)26(35)30-14-15-32-27(33-21-28)31-13-8-18-36-23-10-7-9-22(19-23)20-34-16-5-2-6-17-34/h3-4,7,9-12,19,29H,2,5-6,8,13-18,20H2,1H3,(H,30,35)(H2,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [125I]-aminopotentidine from rat H2R expressed in human COS7 cells incubated for 90 mins gamma counting method |
ACS Med Chem Lett 11: 1521-1528 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00033
BindingDB Entry DOI: 10.7270/Q2W380WD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(RAT) | BDBM50404029
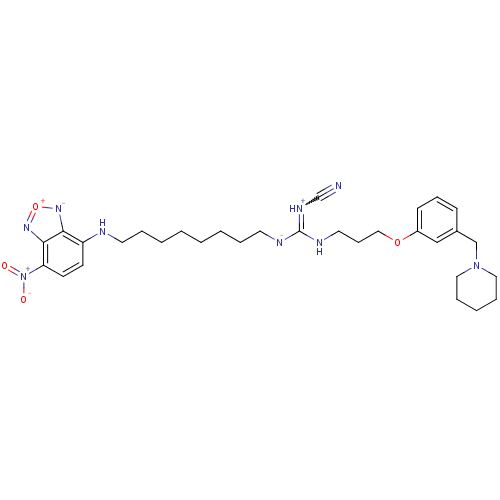
(CHEMBL45728)Show SMILES [O-][N+](=O)c1ccc(NCCCCCCCC[N-]C(NCCCOc2cccc(CN3CCCCC3)c2)=[NH+]C#N)c2[n-][o+]nc12 |w:36.38| Show InChI InChI=1S/C31H42N9O4/c32-24-36-31(35-18-11-21-43-26-13-10-12-25(22-26)23-39-19-8-5-9-20-39)34-17-7-4-2-1-3-6-16-33-27-14-15-28(40(41)42)30-29(27)37-44-38-30/h10,12-15,22,33H,1-9,11,16-21,23H2,(H-,34,35,36)/q-1/p+1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [125I]-aminopotentidine from rat H2R expressed in human COS7 cells incubated for 90 mins gamma counting method |
ACS Med Chem Lett 11: 1521-1528 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00033
BindingDB Entry DOI: 10.7270/Q2W380WD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(RAT) | BDBM50094703
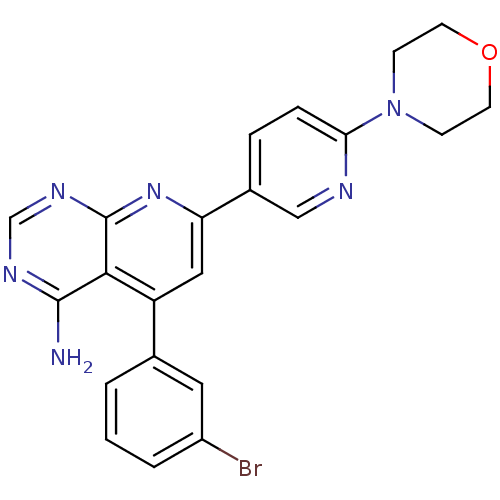
(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(RAT) | BDBM50133817
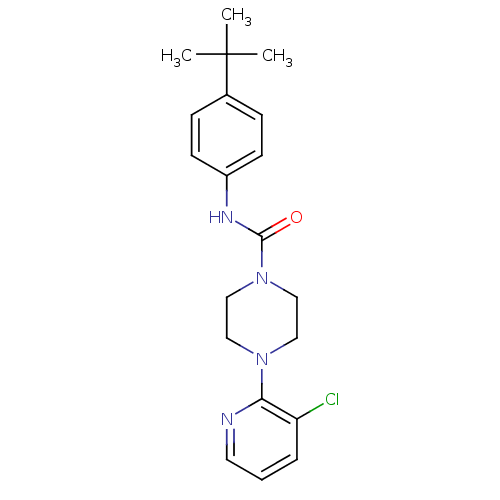
(4-(3-Chloro-pyridin-2-yl)-piperazine-1-carboxylic ...)Show SMILES CC(C)(C)c1ccc(NC(=O)N2CCN(CC2)c2ncccc2Cl)cc1 Show InChI InChI=1S/C20H25ClN4O/c1-20(2,3)15-6-8-16(9-7-15)23-19(26)25-13-11-24(12-14-25)18-17(21)5-4-10-22-18/h4-10H,11-14H2,1-3H3,(H,23,26) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pudue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 377-86 (2003)
Article DOI: 10.1124/jpet.102.045674
BindingDB Entry DOI: 10.7270/Q2TX3CX5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data