

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50103475 (Pmp-Tyr-Ile-Thr-Asn-Cys-Pro-Orn-phe(I,N3)-NH2 | Pm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Ability to inhibit OT-induced inositol phosphate accumulation was determined in CHO cells expressing the human OT receptor | J Med Chem 44: 3022-30 (2001) BindingDB Entry DOI: 10.7270/Q29S1Q9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50043198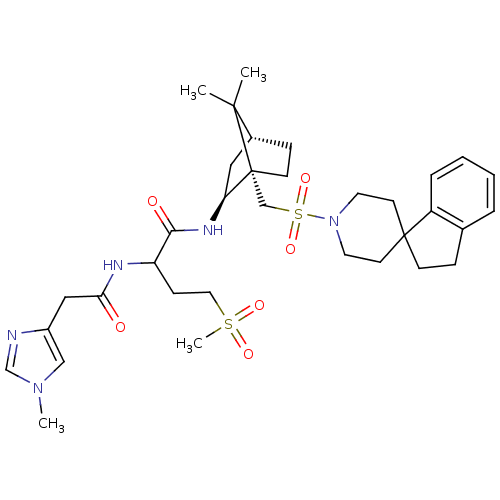 (1N-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of radioligand [3H]-oxytocin binding at the oxytocin (OT) receptor in rat uterine tissue | J Med Chem 36: 3993-4005 (1994) BindingDB Entry DOI: 10.7270/Q27M070W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50100381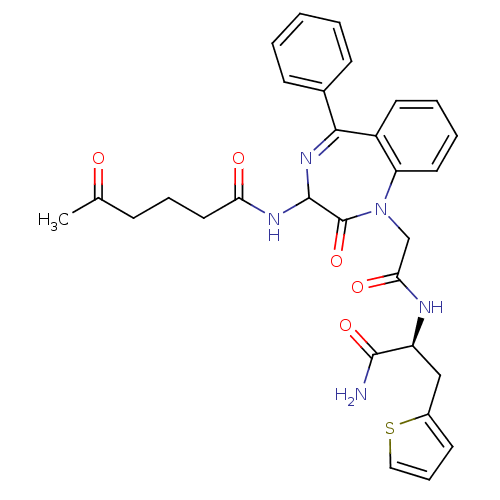 (5-Oxo-hexanoic acid {1-[((S)-1-carbamoyl-2-thiophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory concentration required for antagonist activity against oxytocin receptor | Bioorg Med Chem Lett 11: 1297-300 (2001) BindingDB Entry DOI: 10.7270/Q2T72GQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50043186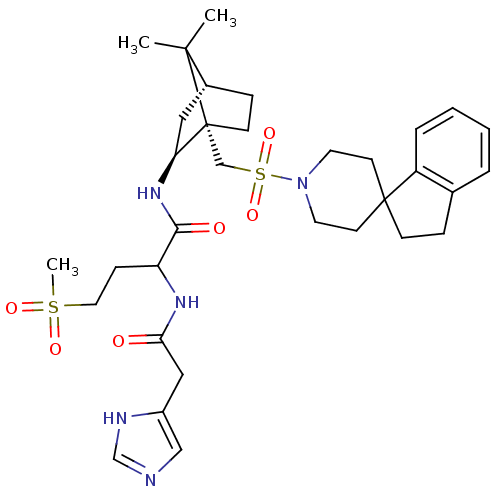 (1N-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of radioligand [3H]-oxytocin binding at the oxytocin (OT) receptor in rat uterine tissue | J Med Chem 36: 3993-4005 (1994) BindingDB Entry DOI: 10.7270/Q27M070W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50407295 (CHEMBL2112895) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-OT from binding to oxytocin receptor of rat uterus | J Med Chem 37: 565-71 (1994) BindingDB Entry DOI: 10.7270/Q21C1VZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50407292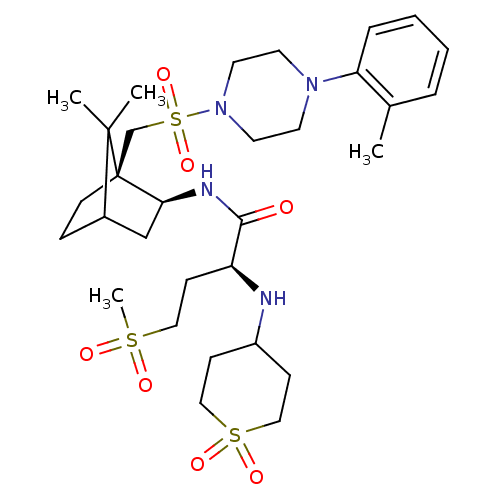 (CHEMBL2112893) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-OT from binding to oxytocin receptor of rat uterus | J Med Chem 37: 565-71 (1994) BindingDB Entry DOI: 10.7270/Q21C1VZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50043130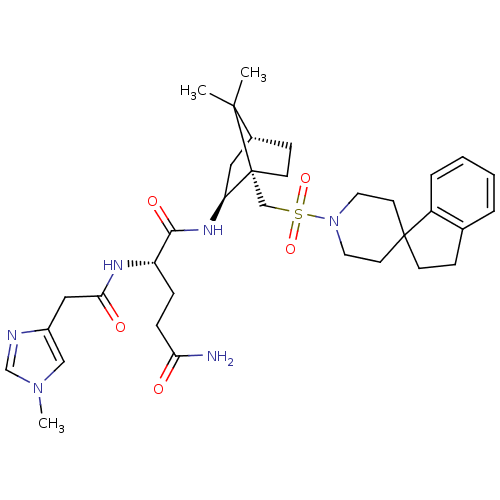 (1N-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of radioligand [3H]-oxytocin binding at the oxytocin (OT) receptor in rat uterine tissue | J Med Chem 36: 3993-4005 (1994) BindingDB Entry DOI: 10.7270/Q27M070W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50043156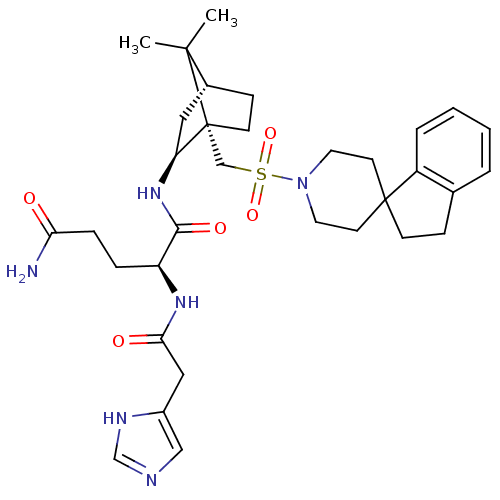 (1N-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of radioligand [3H]-oxytocin binding at the oxytocin (OT) receptor in rat uterine tissue | J Med Chem 36: 3993-4005 (1994) BindingDB Entry DOI: 10.7270/Q27M070W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50406692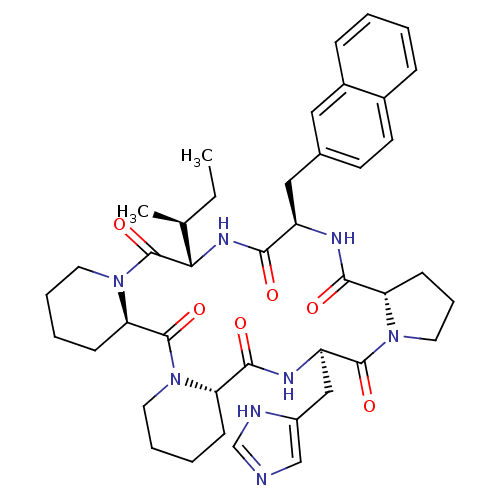 (CHEMBL2112249) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required to displace 50% of [3H]oxytocin from rat uterine receptor. | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50043155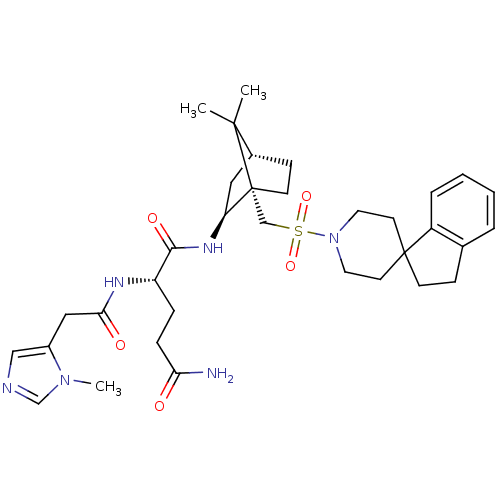 (1N-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of radioligand [3H]-oxytocin binding at the oxytocin (OT) receptor in rat uterine tissue | J Med Chem 36: 3993-4005 (1994) BindingDB Entry DOI: 10.7270/Q27M070W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50407294 (CHEMBL2112897) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-OT from binding to oxytocin receptor of rat uterus | J Med Chem 37: 565-71 (1994) BindingDB Entry DOI: 10.7270/Q21C1VZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50043080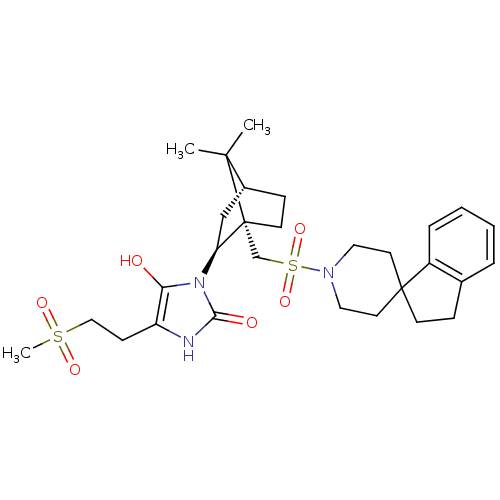 (3-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of radioligand [3H]-oxytocin binding at the oxytocin (OT) receptor in rat uterine tissue | J Med Chem 36: 3993-4005 (1994) BindingDB Entry DOI: 10.7270/Q27M070W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50205990 (CHEMBL395429 | OXYTOCIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Patras Curated by ChEMBL | Assay Description Displacement of [3H]OT from human OT receptor expressed in HEK cells | Eur J Med Chem 42: 799-806 (2007) Article DOI: 10.1016/j.ejmech.2006.12.016 BindingDB Entry DOI: 10.7270/Q2P55N5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50407289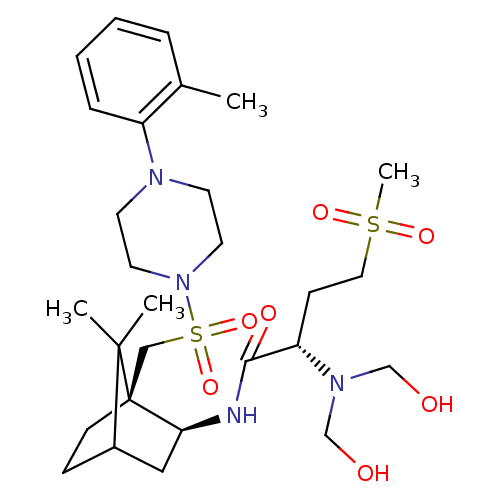 (CHEMBL2112901) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-OT from binding to oxytocin receptor of rat uterus | J Med Chem 37: 565-71 (1994) BindingDB Entry DOI: 10.7270/Q21C1VZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50043114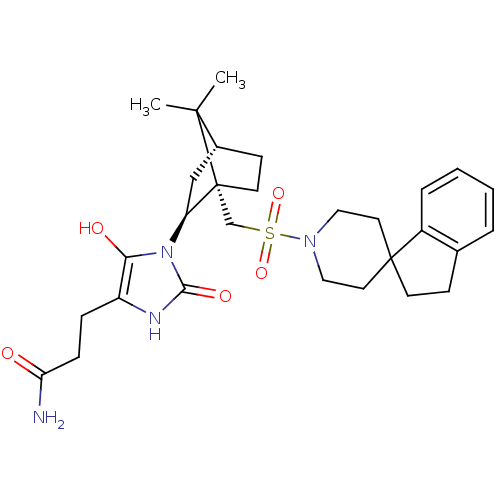 (3-{1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of radioligand [3H]-oxytocin binding at the oxytocin (OT) receptor in rat uterine tissue | J Med Chem 36: 3993-4005 (1994) BindingDB Entry DOI: 10.7270/Q27M070W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50190528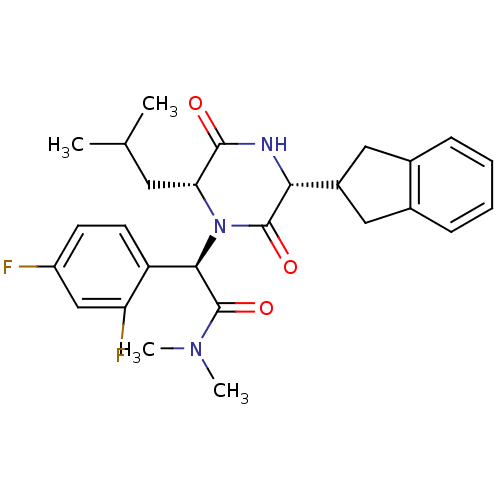 ((2R)-2-(2,4-difluorophenyl)-2-[(3R,6R)-3-(2,3-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiovascular and Urogenital Centre of Excellence for Drug Discovery Curated by ChEMBL | Assay Description Agonist activity at human OTR expressed in CHO cells assessed as inhibition of oxytocin-induced calcium mobilization by FLIPR assay | J Med Chem 49: 4159-70 (2006) Article DOI: 10.1021/jm060073e BindingDB Entry DOI: 10.7270/Q269735Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50043102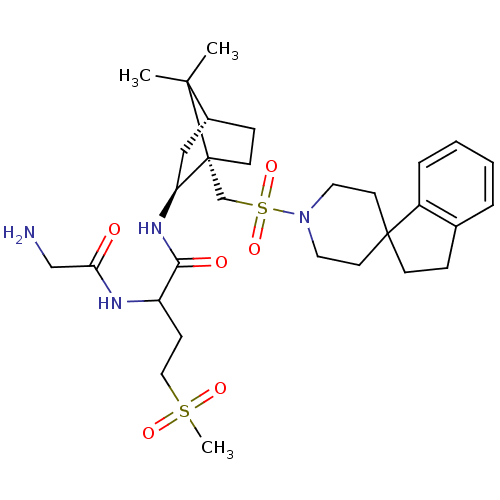 (1N-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of radioligand [3H]-oxytocin binding at the oxytocin (OT) receptor in rat uterine tissue | J Med Chem 36: 3993-4005 (1994) BindingDB Entry DOI: 10.7270/Q27M070W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50043174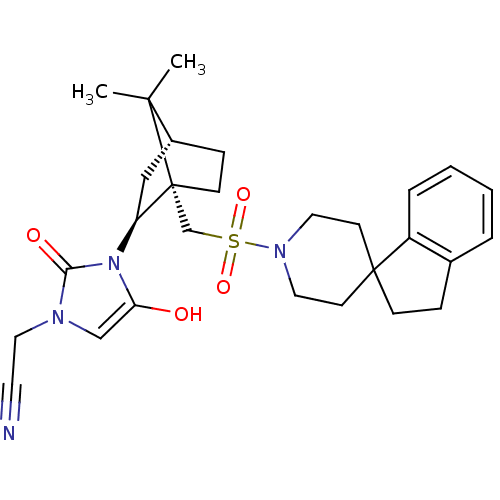 (2-{3-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of radioligand [3H]-oxytocin binding at the oxytocin (OT) receptor in rat uterine tissue | J Med Chem 36: 3993-4005 (1994) BindingDB Entry DOI: 10.7270/Q27M070W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50559938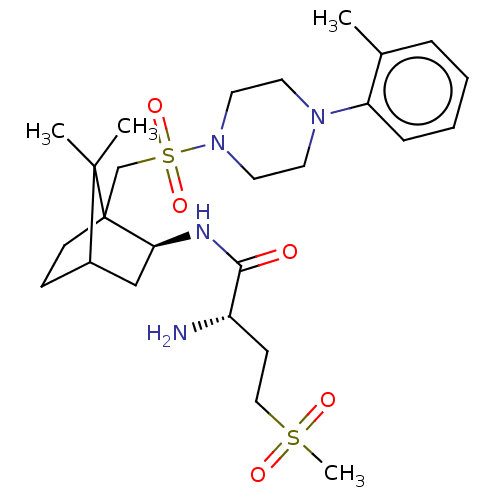 (CHEMBL154668) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human OXTR expressed in CHO-K1 cells by PathHunter beta-arrestin assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115546 BindingDB Entry DOI: 10.7270/Q2F193D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50043098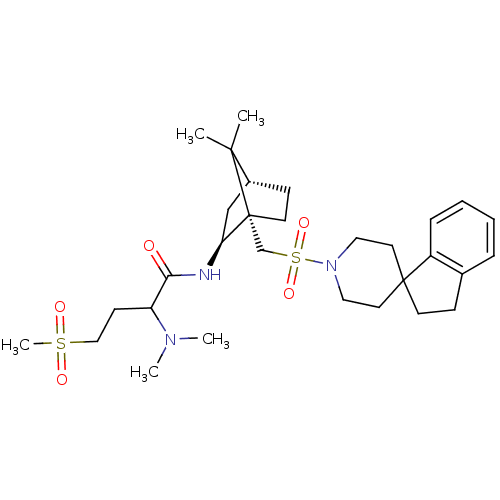 (1N-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of radioligand [3H]-oxytocin binding at the oxytocin (OT) receptor in rat uterine tissue | J Med Chem 36: 3993-4005 (1994) BindingDB Entry DOI: 10.7270/Q27M070W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50407290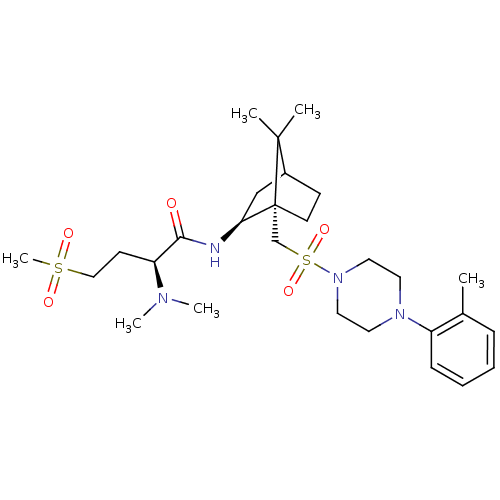 (CHEMBL2112896) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-OT from binding to oxytocin receptor of rat uterus | J Med Chem 37: 565-71 (1994) BindingDB Entry DOI: 10.7270/Q21C1VZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50100380 (5-Oxo-hexanoic acid (1-{[(S)-1-carbamoyl-2-(3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory concentration required for antagonist activity against oxytocin receptor | Bioorg Med Chem Lett 11: 1297-300 (2001) BindingDB Entry DOI: 10.7270/Q2T72GQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50043086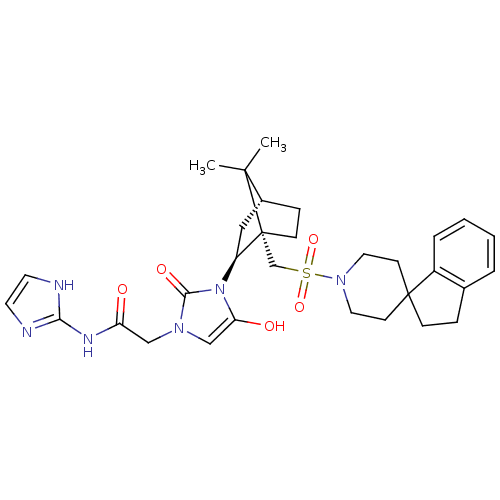 (1N-(1H-2-imidazolyl)-2-{3-[7,7-dimethyl-1-spiro[2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of radioligand [3H]-oxytocin binding at the oxytocin (OT) receptor in rat uterine tissue | J Med Chem 36: 3993-4005 (1994) BindingDB Entry DOI: 10.7270/Q27M070W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50043120 (1N-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of radioligand [3H]-oxytocin binding at the oxytocin (OT) receptor in rat uterine tissue | J Med Chem 36: 3993-4005 (1994) BindingDB Entry DOI: 10.7270/Q27M070W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM81894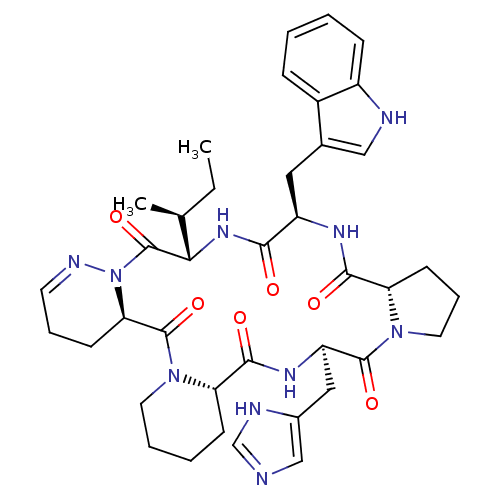 (Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required to displace 50% of [3H]oxytocin from rat uterine receptor. | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50043060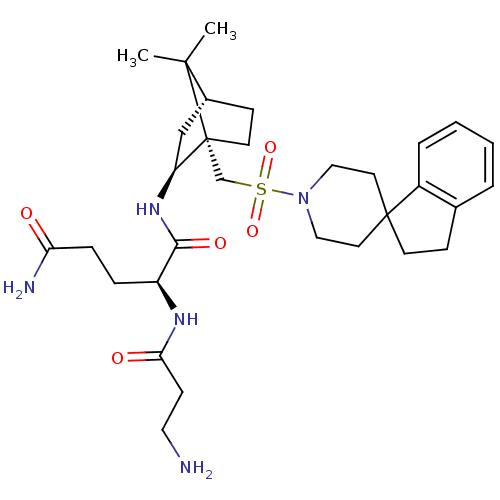 (1N-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of radioligand [3H]-oxytocin binding at the oxytocin (OT) receptor in rat uterine tissue | J Med Chem 36: 3993-4005 (1994) BindingDB Entry DOI: 10.7270/Q27M070W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50043141 (5-(3-amino-1H-1,2,4-triazol-5-ylaminomethyl)-3-[7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of radioligand [3H]-oxytocin binding at the oxytocin (OT) receptor in rat uterine tissue | J Med Chem 36: 3993-4005 (1994) BindingDB Entry DOI: 10.7270/Q27M070W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410616 (CHEMBL2113185) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human Oxytocin induced intracellular Calcium mobilization in human Oxytocin receptor transfected HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410619 (CHEMBL2113208) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human Oxytocin induced intracellular Calcium mobilization in human Oxytocin receptor transfected HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50205997 (CHEMBL395290 | [Pip7]OT) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Patras Curated by ChEMBL | Assay Description Displacement of [3H]OT from human OT receptor expressed in HEK cells | Eur J Med Chem 42: 799-806 (2007) Article DOI: 10.1016/j.ejmech.2006.12.016 BindingDB Entry DOI: 10.7270/Q2P55N5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50285308 (3-amino-1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-ind...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor | Bioorg Med Chem Lett 5: 119-122 (1995) Article DOI: 10.1016/0960-894X(94)00469-V BindingDB Entry DOI: 10.7270/Q2QF8STX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50043143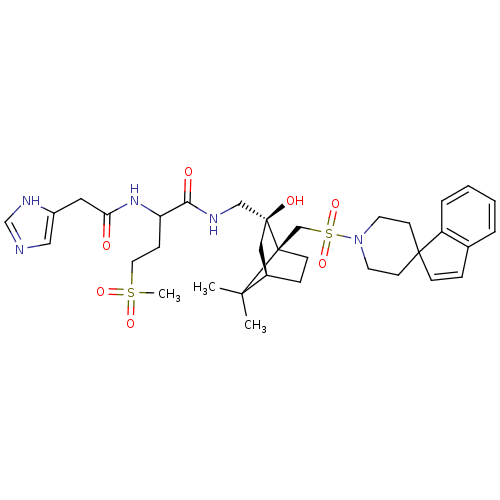 (1N-[2-hydroxy-7,7-dimethyl-1-spiro[1H-indene-1,4'-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of radioligand [3H]-oxytocin binding at the oxytocin (OT) receptor in rat uterine tissue | J Med Chem 36: 3993-4005 (1994) BindingDB Entry DOI: 10.7270/Q27M070W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50027070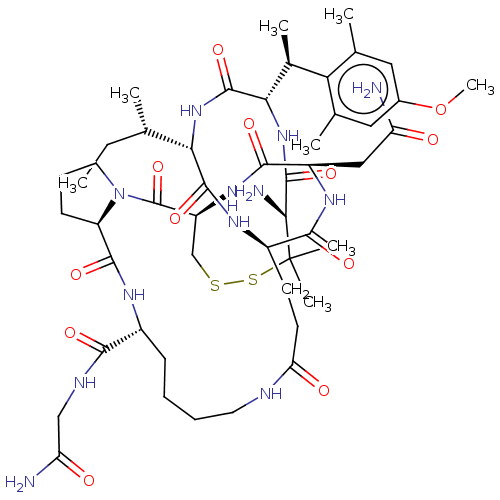 (CHEMBL2369136) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards oxytocin receptor | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410635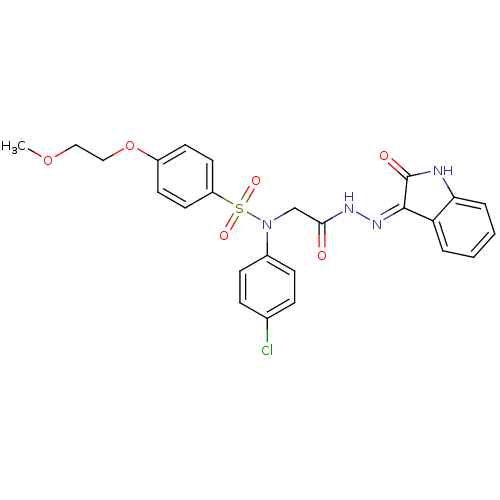 (CHEMBL2113211) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human Oxytocin induced intracellular Calcium mobilization in human Oxytocin receptor transfected HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50043204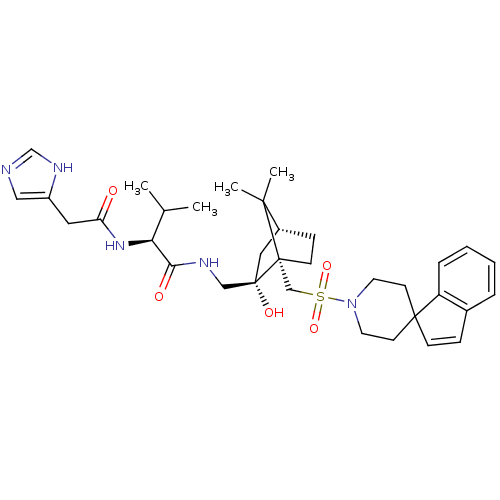 (1N-[2-hydroxy-7,7-dimethyl-1-spiro[1H-indene-1,4'-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of radioligand [3H]-oxytocin binding at the oxytocin (OT) receptor in rat uterine tissue | J Med Chem 36: 3993-4005 (1994) BindingDB Entry DOI: 10.7270/Q27M070W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50406697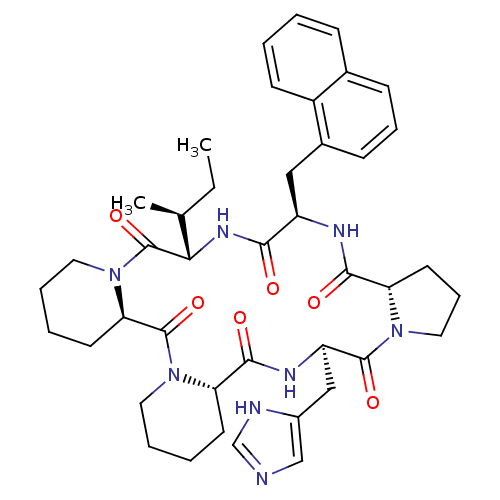 (CHEMBL2112250) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required to displace 50% of [3H]oxytocin from rat uterine receptor. | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50001311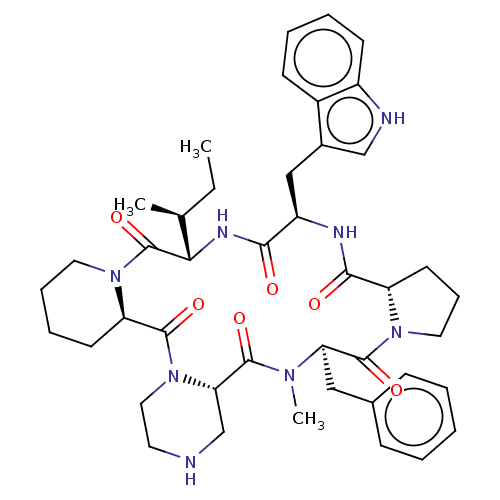 (24-benzyl-16-(1H-3-indolylmethyl)-25-methyl-13-[1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from pregnant rats | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50326719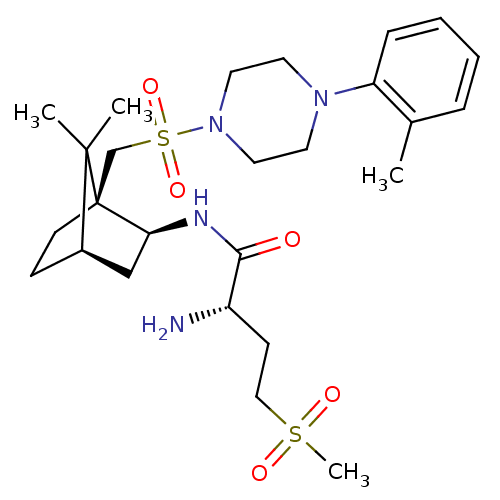 ((S)-2-amino-N-((1S,2S,4R)-7,7-dimethyl-1-((4-o-tol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Yerkes National Primate Research Center Curated by ChEMBL | Assay Description Inhibition of rat uterus OT receptor | Bioorg Med Chem Lett 23: 902-6 (2013) Article DOI: 10.1016/j.bmcl.2012.10.116 BindingDB Entry DOI: 10.7270/Q29Z9690 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50326719 ((S)-2-amino-N-((1S,2S,4R)-7,7-dimethyl-1-((4-o-tol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-OT from binding to oxytocin receptor of rat uterus | J Med Chem 37: 565-71 (1994) BindingDB Entry DOI: 10.7270/Q21C1VZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50285295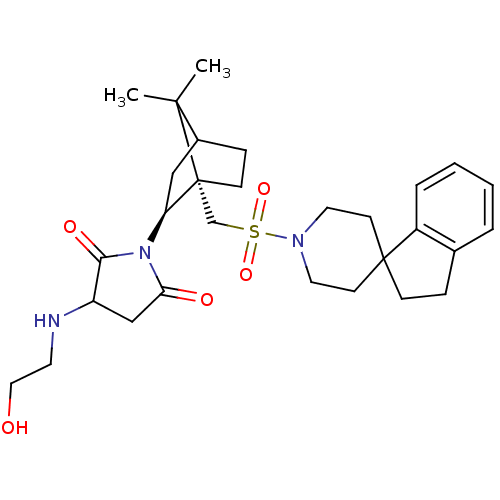 (1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor | Bioorg Med Chem Lett 5: 119-122 (1995) Article DOI: 10.1016/0960-894X(94)00469-V BindingDB Entry DOI: 10.7270/Q2QF8STX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50407293 (CHEMBL2112898) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-OT from binding to oxytocin receptor of rat uterus | J Med Chem 37: 565-71 (1994) BindingDB Entry DOI: 10.7270/Q21C1VZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50435057 (CHEMBL2391300) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Yerkes National Primate Research Center Curated by ChEMBL | Assay Description Inhibition of rat uterus OT receptor | Bioorg Med Chem Lett 23: 902-6 (2013) Article DOI: 10.1016/j.bmcl.2012.10.116 BindingDB Entry DOI: 10.7270/Q29Z9690 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50384820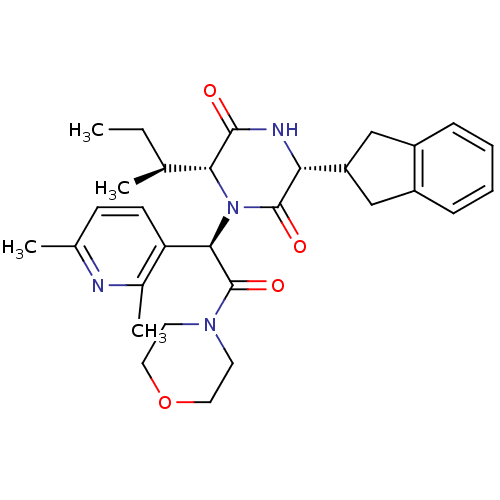 (EPELSIBAN | GSK557296B) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical CO., LTD. Curated by ChEMBL | Assay Description Antagonist activity at human OTR expressed in HEK293 cells assessed as decrease in calcium flux measured after 10 mins in presence of vasopressin by ... | ACS Med Chem Lett 10: 996-1001 (2019) Article DOI: 10.1021/acsmedchemlett.9b00186 BindingDB Entry DOI: 10.7270/Q2Q52T0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50516419 (CHEMBL4464246) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical CO., LTD. Curated by ChEMBL | Assay Description Antagonist activity at human OTR expressed in HEK293 cells assessed as decrease in calcium flux measured after 10 mins in presence of vasopressin by ... | ACS Med Chem Lett 10: 996-1001 (2019) Article DOI: 10.1021/acsmedchemlett.9b00186 BindingDB Entry DOI: 10.7270/Q2Q52T0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50516428 (CHEMBL4449435) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical CO., LTD. Curated by ChEMBL | Assay Description Antagonist activity at human OTR expressed in HEK293 cells assessed as decrease in calcium flux measured after 10 mins in presence of vasopressin by ... | ACS Med Chem Lett 10: 996-1001 (2019) Article DOI: 10.1021/acsmedchemlett.9b00186 BindingDB Entry DOI: 10.7270/Q2Q52T0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50406698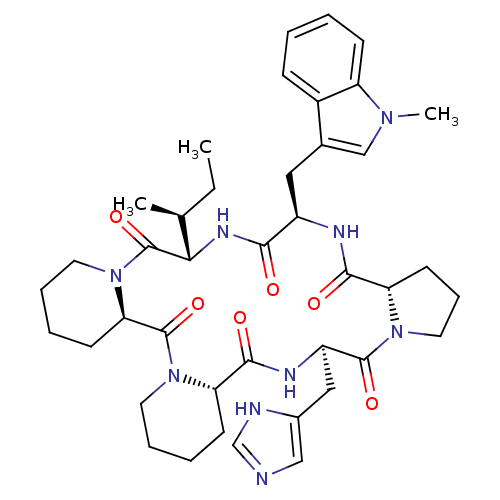 (CHEMBL2112247) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required to displace 50% of [3H]oxytocin from rat uterine receptor. | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50285293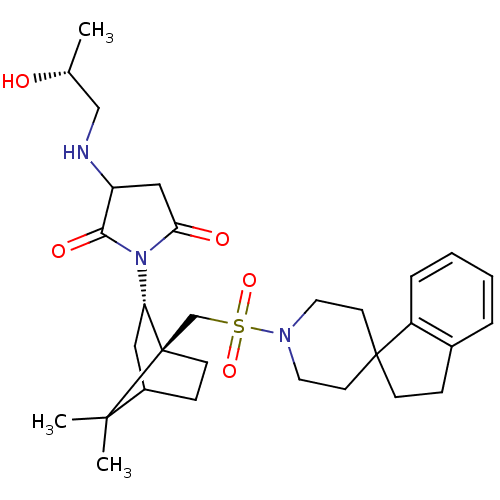 (1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor | Bioorg Med Chem Lett 5: 119-122 (1995) Article DOI: 10.1016/0960-894X(94)00469-V BindingDB Entry DOI: 10.7270/Q2QF8STX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50043160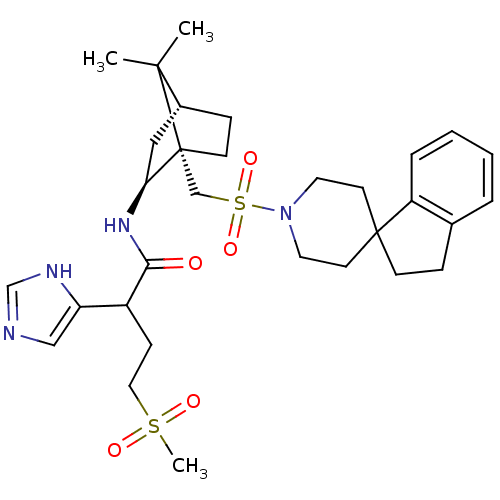 (1N-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of radioligand [3H]-AVP binding to the vasopressin V2 receptor in rat kidney tissue | J Med Chem 36: 3993-4005 (1994) BindingDB Entry DOI: 10.7270/Q27M070W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50043109 (1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of radioligand [3H]-oxytocin binding at the oxytocin (OT) receptor in rat uterine tissue | J Med Chem 36: 3993-4005 (1994) BindingDB Entry DOI: 10.7270/Q27M070W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50043160 (1N-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of radioligand [3H]oxytocin binding at the oxytocin (OT) receptor in rat uterine tissue | J Med Chem 36: 3993-4005 (1994) BindingDB Entry DOI: 10.7270/Q27M070W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 606 total ) | Next | Last >> |