

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM50469330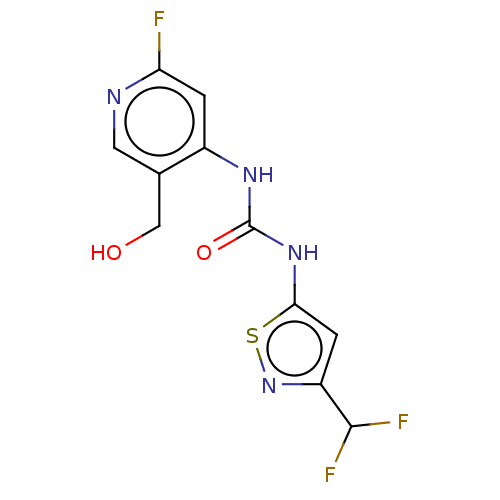 (CHEMBL4295096) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant His10-tagged ZZ-HCV3C-BRM ATPase-SnAC (636 to 1331 residues) (unknown origin) expressed in insect sf9 cells preincubated fo... | J Med Chem 61: 10155-10172 (2018) Article DOI: 10.1021/acs.jmedchem.8b01318 BindingDB Entry DOI: 10.7270/Q2TH8QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM50469324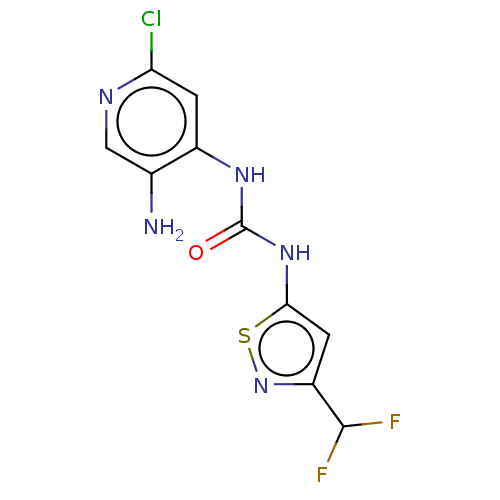 (CHEMBL4286345) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant His10-tagged ZZ-HCV3C-BRM ATPase-SnAC (636 to 1331 residues) (unknown origin) expressed in insect sf9 cells preincubated fo... | J Med Chem 61: 10155-10172 (2018) Article DOI: 10.1021/acs.jmedchem.8b01318 BindingDB Entry DOI: 10.7270/Q2TH8QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM50469320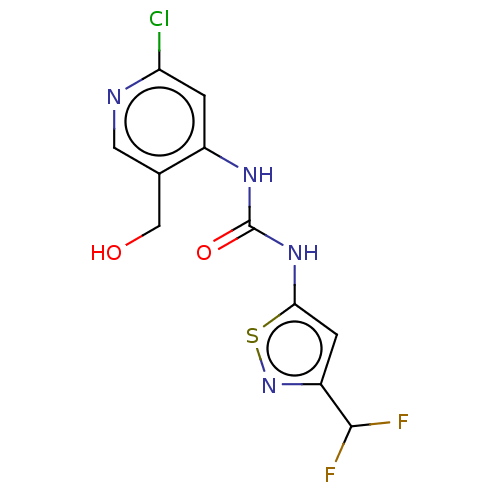 (CHEMBL4293567) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant His10-tagged ZZ-HCV3C-BRM ATPase-SnAC (636 to 1331 residues) (unknown origin) expressed in insect sf9 cells preincubated fo... | J Med Chem 61: 10155-10172 (2018) Article DOI: 10.1021/acs.jmedchem.8b01318 BindingDB Entry DOI: 10.7270/Q2TH8QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM50469331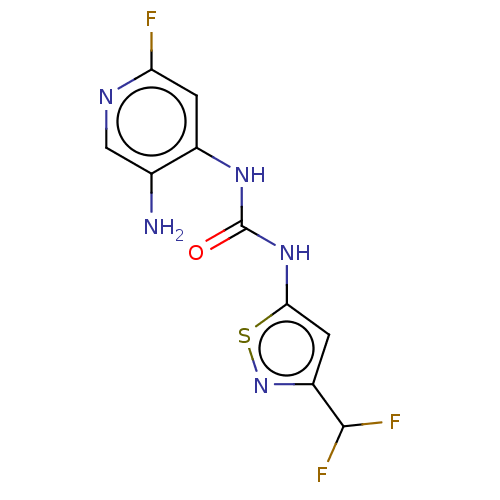 (CHEMBL4282980) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant His10-tagged ZZ-HCV3C-BRM ATPase-SnAC (636 to 1331 residues) (unknown origin) expressed in insect sf9 cells preincubated fo... | J Med Chem 61: 10155-10172 (2018) Article DOI: 10.1021/acs.jmedchem.8b01318 BindingDB Entry DOI: 10.7270/Q2TH8QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM50469329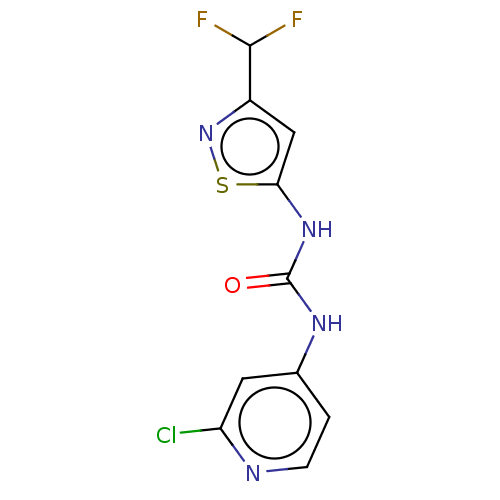 (CHEMBL4278436) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant His10-tagged ZZ-HCV3C-BRM ATPase-SnAC (636 to 1331 residues) (unknown origin) expressed in insect sf9 cells preincubated fo... | J Med Chem 61: 10155-10172 (2018) Article DOI: 10.1021/acs.jmedchem.8b01318 BindingDB Entry DOI: 10.7270/Q2TH8QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394585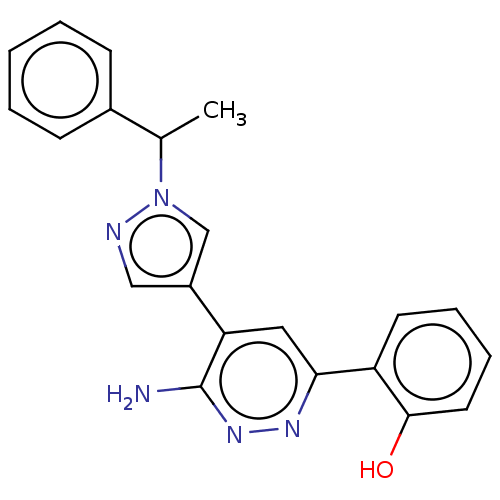 (2-(6-amino-5-(1-(1- | US10308614, Example 188) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394575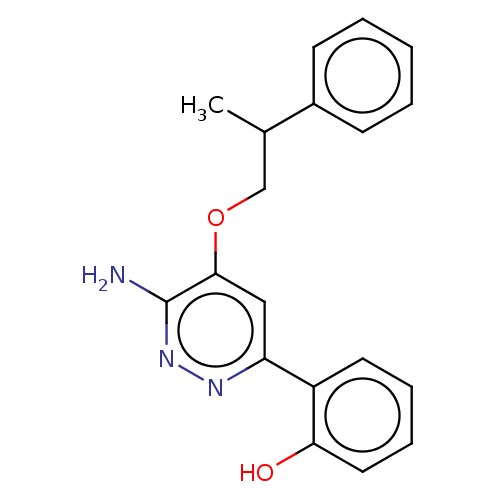 (2-(6-amino-5-(2- | US10308614, Example 178) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394605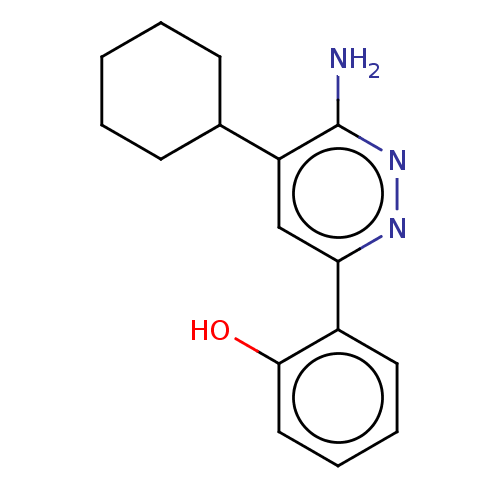 (2-(6-amino-5-cyclohexylpyridazin-3-yl)phenol | US1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM50469325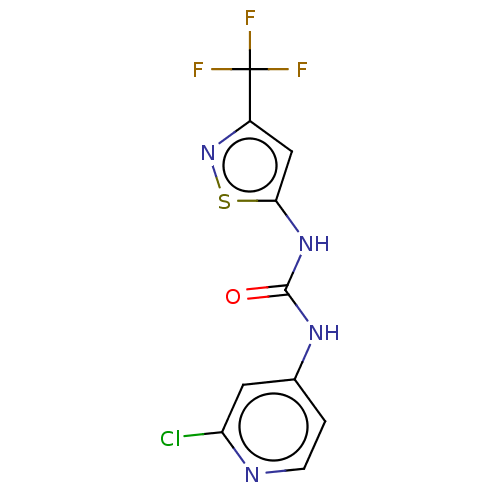 (CHEMBL4294655) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant His10-tagged ZZ-HCV3C-BRM ATPase-SnAC (636 to 1331 residues) (unknown origin) expressed in insect sf9 cells preincubated fo... | J Med Chem 61: 10155-10172 (2018) Article DOI: 10.1021/acs.jmedchem.8b01318 BindingDB Entry DOI: 10.7270/Q2TH8QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394584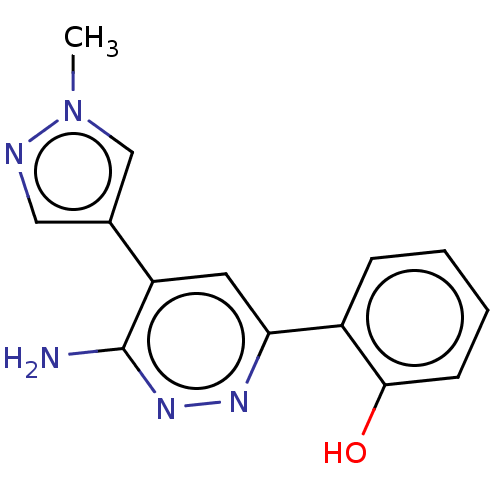 (2-(6-amino-5-(1-methyl- | US10308614, Example 187) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394567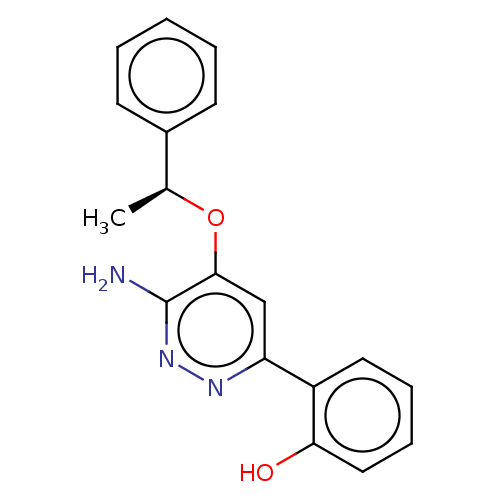 ((S)-2-(6-amino-5-(1- | US10308614, Example 170) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14.9 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394528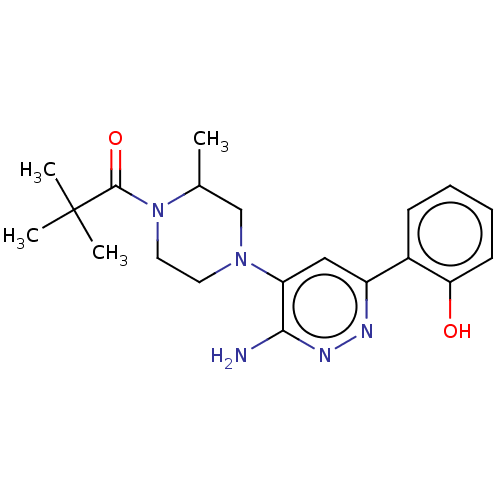 (1-(4-(3-amino-6-(2- | US10308614, Example 129) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18.4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394571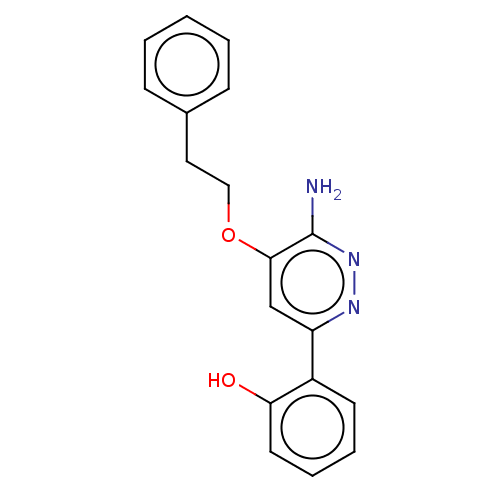 (2-(6-amino-5- | US10308614, Example 174) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394403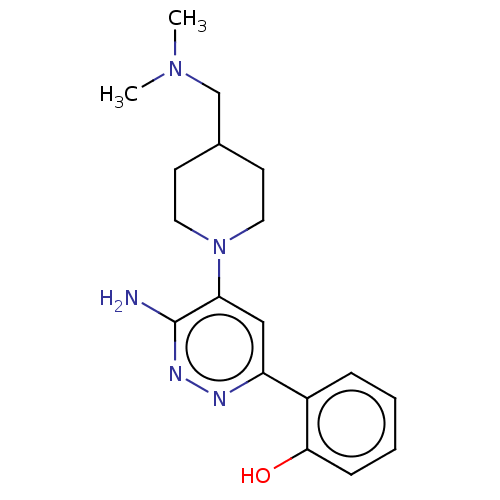 (2-(6-amino-5-(4-((dimethylami- | US10308614, Examp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394606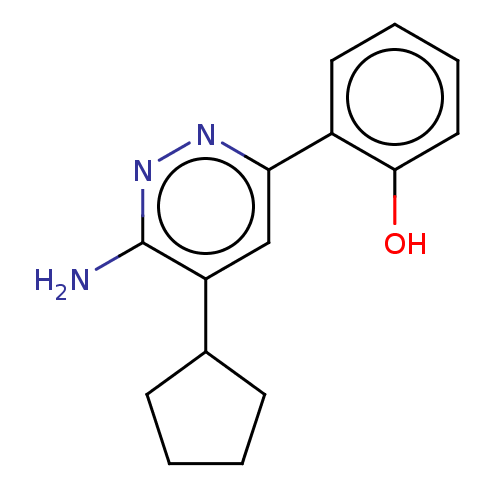 (2-(6-amino-5- | US10308614, Example 210) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM50469321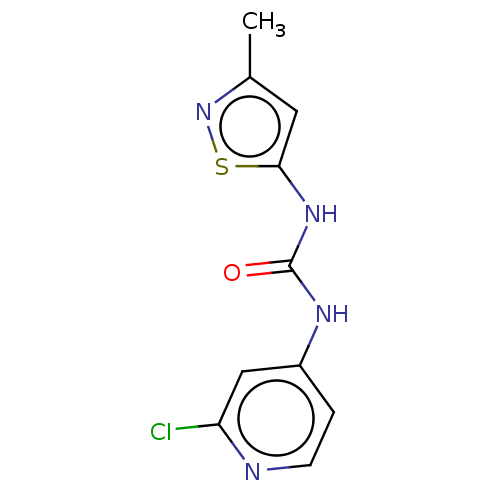 (CHEMBL4286757) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant His10-tagged ZZ-HCV3C-BRM ATPase-SnAC (636 to 1331 residues) (unknown origin) expressed in insect sf9 cells preincubated fo... | J Med Chem 61: 10155-10172 (2018) Article DOI: 10.1021/acs.jmedchem.8b01318 BindingDB Entry DOI: 10.7270/Q2TH8QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394456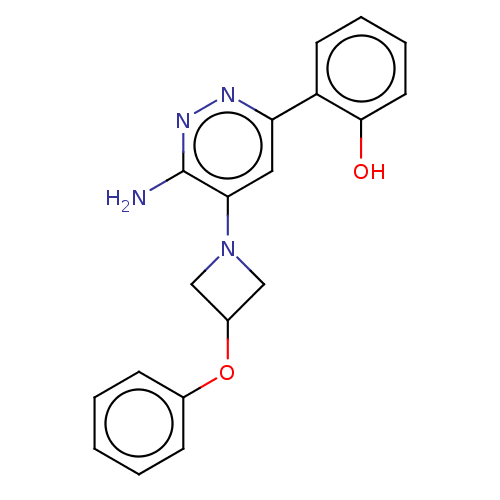 (2-(6-amino-5-(3- | US10308614, Example 57) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 35.6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394507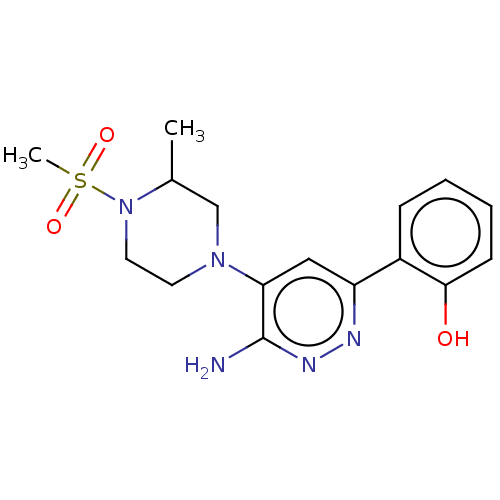 (2-(6-amino-5-(3-methyl-4-(methylsulfonyl)piperazin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 35.8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394414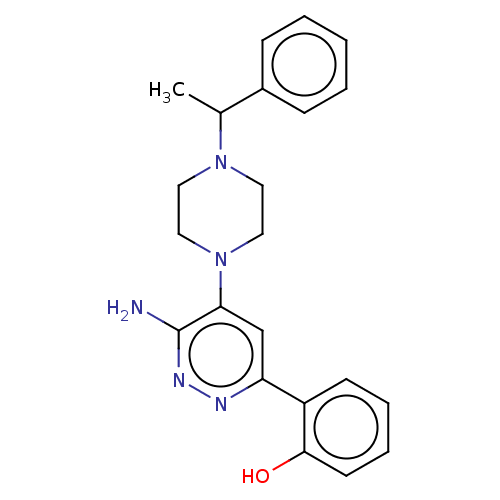 (2-(6-amino-5-(4-(1- | US10308614, Example 15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 36.1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM394583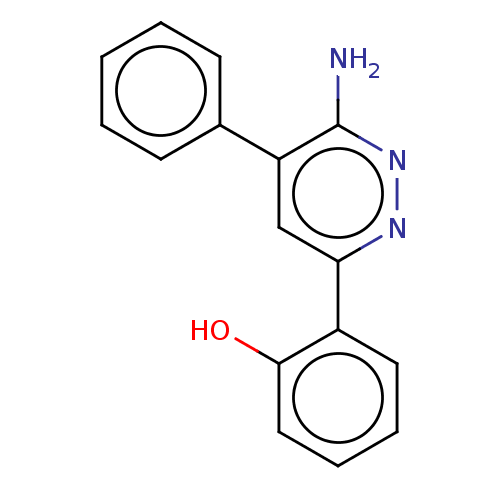 (2-(6-amino-5-phenylpyridazin-3-yl)phenol | US10308...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00662 BindingDB Entry DOI: 10.7270/Q2TB1BX4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394583 (2-(6-amino-5-phenylpyridazin-3-yl)phenol | US10308...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 37.3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394521 (1-(4-(3-amino-6-(2-hydroxyphenyl)pyridazin-4-yl)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394614 (2-(6-amino-5-(1-benzylpiperidin-4-yl)pyridazin-3-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 39.3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394490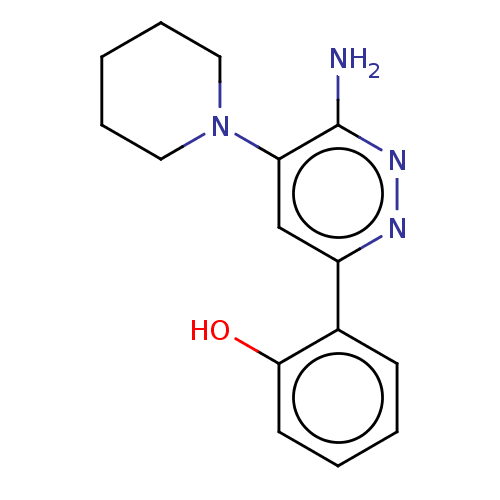 (2-(6-amino-5-(piperidin-1-yl)pyridazin-3-yl)phenol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 45.9 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394518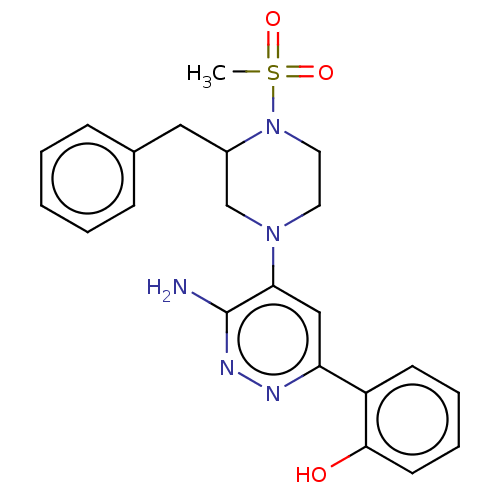 (2-(6-amino-5-(3-benzyl- | US10308614, Example 119) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 46.8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394408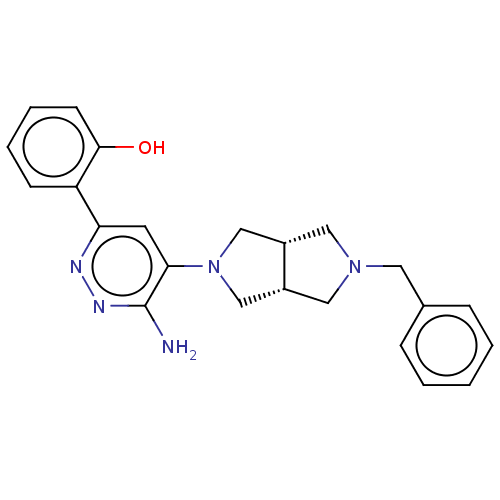 (2-(6-amino-5-((3aR,6aS)-5- | US10308614, Example 9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 47.6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394586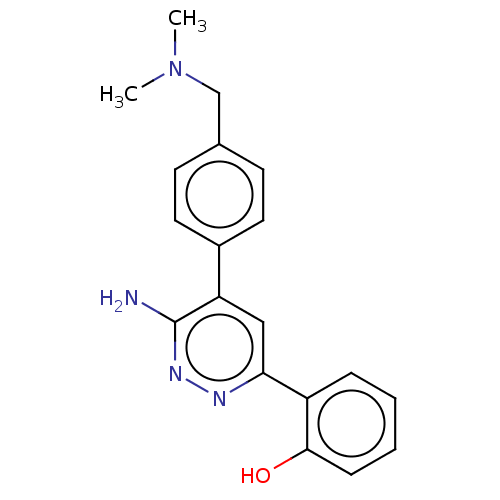 (2-(6-amino-5-(4- | US10308614, Example 189) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 49.3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394577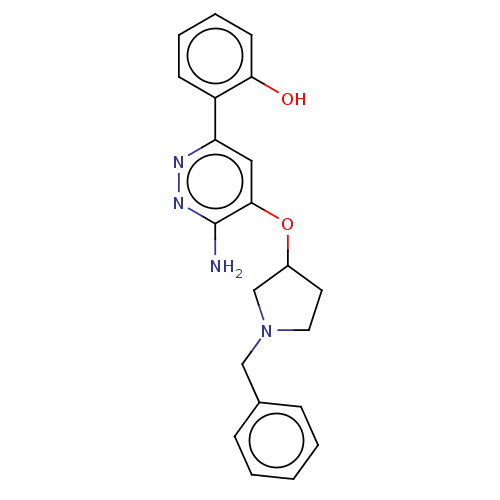 (2-(6-amino-5-((1- | US10308614, Example 180) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 59.7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM50469333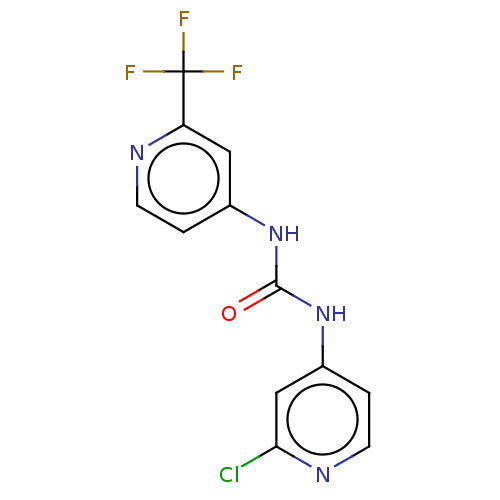 (CHEMBL4290175) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant His10-tagged ZZ-HCV3C-BRM ATPase-SnAC (636 to 1331 residues) (unknown origin) expressed in insect sf9 cells preincubated fo... | J Med Chem 61: 10155-10172 (2018) Article DOI: 10.1021/acs.jmedchem.8b01318 BindingDB Entry DOI: 10.7270/Q2TH8QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394401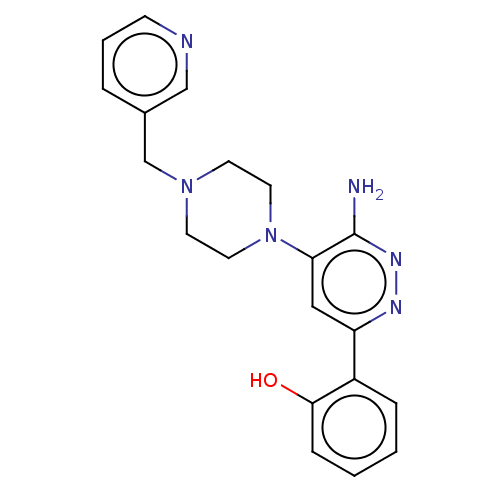 (2-(6-amino-5-(4-(pyridin- | US10308614, Example 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60.7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394432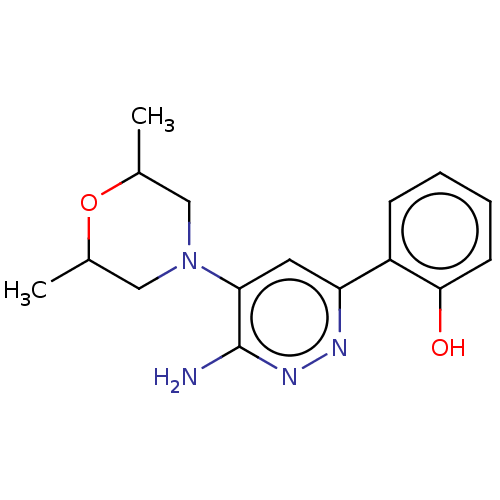 (2-(6-amino-5-(2,6- | US10308614, Example 33) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 69.6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394444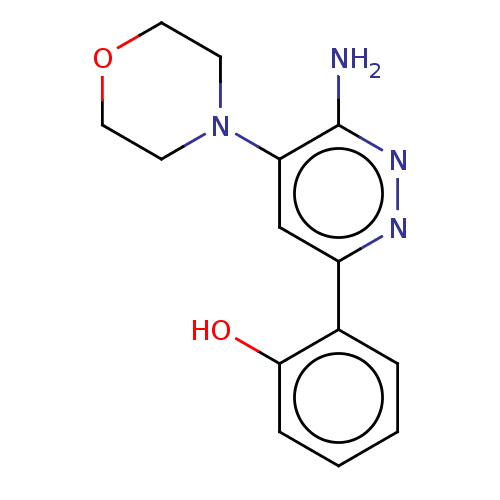 (2-(6-amino-5- | US10308614, Example 45) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 71.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394402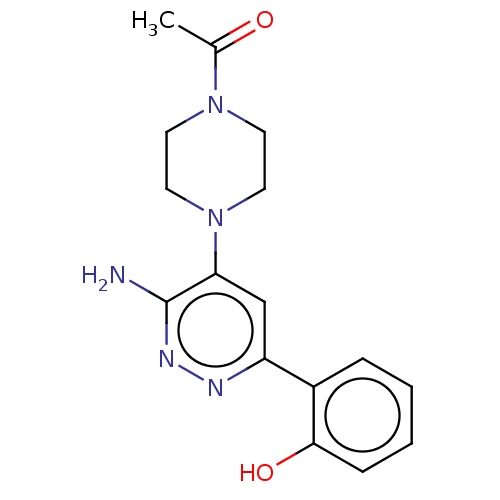 (1-(4-(3-amino-6-(2- | US10308614, Example 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 71.7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394415 (2-(6-amino-5-(8-benzyl-3,8- | US10308614, Example ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394574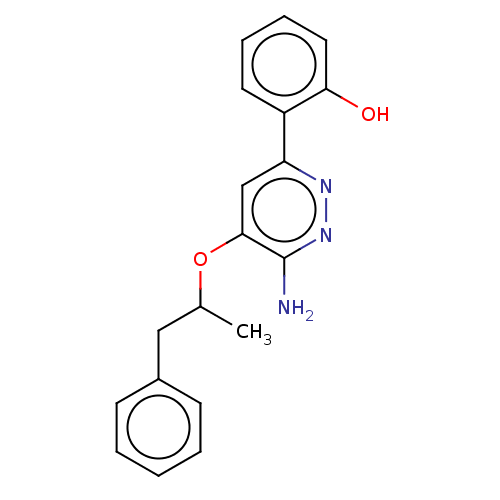 (2-(6-amino-5-((1- | US10308614, Example 177) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 92.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM646010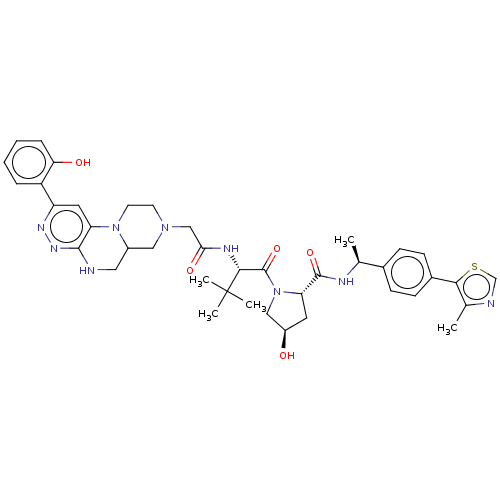 ((2S,4R)-4-hydroxy-1-((2S)-2-(2-(2-(2- hydroxypheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM646016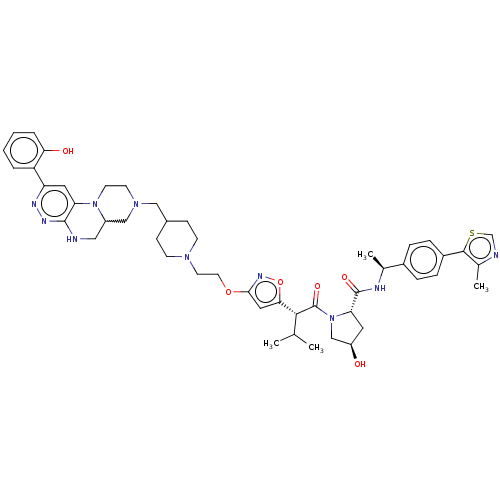 ((2S,4R)-4-hydroxy-1-((2S)-2-(3-(2-(4-((2-(2- hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM646014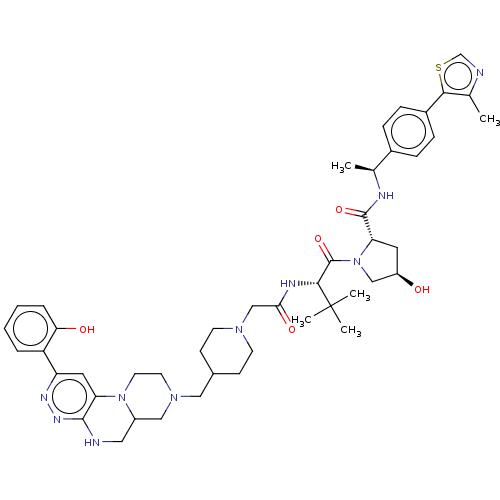 ((2S,4R)-4-hydroxy-1-((2S)-2-(2-(4-((2-(2- hydroxyp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM646012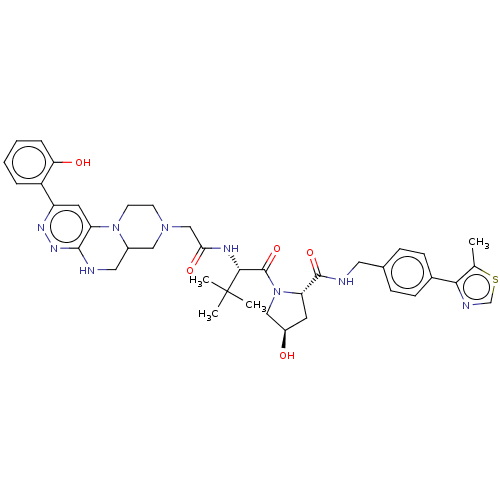 ((2S,4R)-4-hydroxy-N-[[4-(5-methyl-1,3- thiazol-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM646015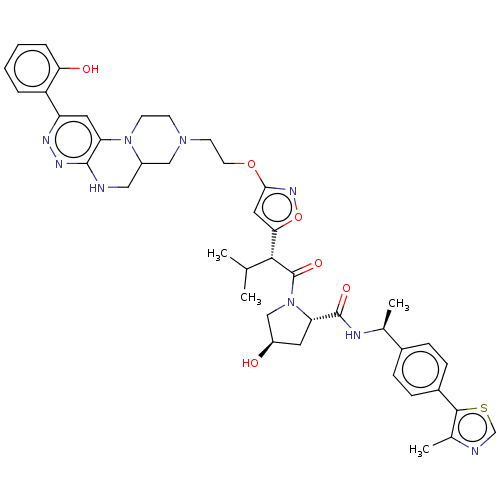 ((2S,4R)-4-hydroxy-1-((2R)-2-(3-(2-(2-(2- hydroxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM50469326 (CHEMBL4279505) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant His10-tagged ZZ-HCV3C-BRM ATPase-SnAC (636 to 1331 residues) (unknown origin) expressed in insect sf9 cells preincubated fo... | J Med Chem 61: 10155-10172 (2018) Article DOI: 10.1021/acs.jmedchem.8b01318 BindingDB Entry DOI: 10.7270/Q2TH8QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394565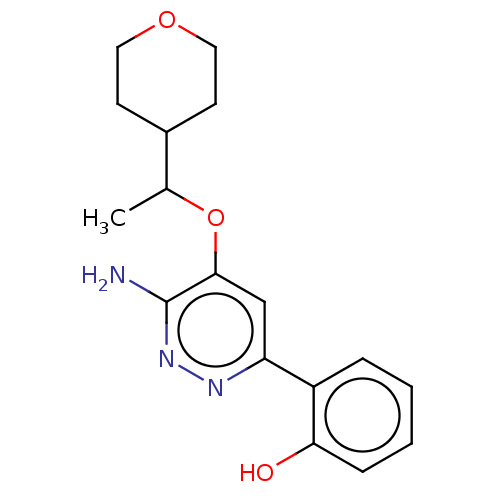 (2-(6-amino-5-(1- | US10308614, Example 168) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 197 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM50469322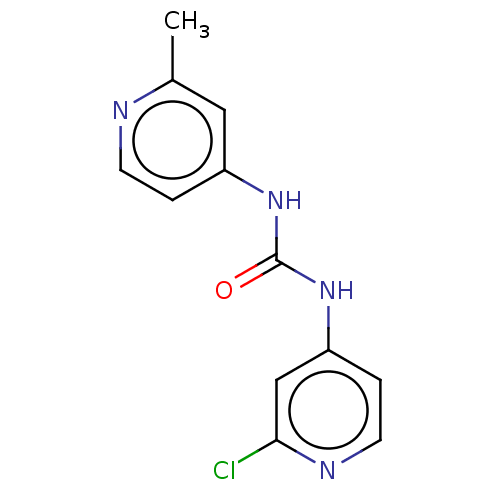 (CHEMBL4281368) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant His10-tagged ZZ-HCV3C-BRM ATPase-SnAC (636 to 1331 residues) (unknown origin) expressed in insect sf9 cells preincubated fo... | J Med Chem 61: 10155-10172 (2018) Article DOI: 10.1021/acs.jmedchem.8b01318 BindingDB Entry DOI: 10.7270/Q2TH8QDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394570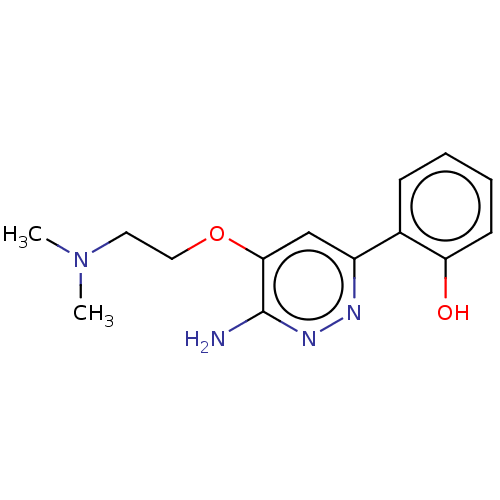 (2-(6-amino-5-(2- | US10308614, Example 173) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 276 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394602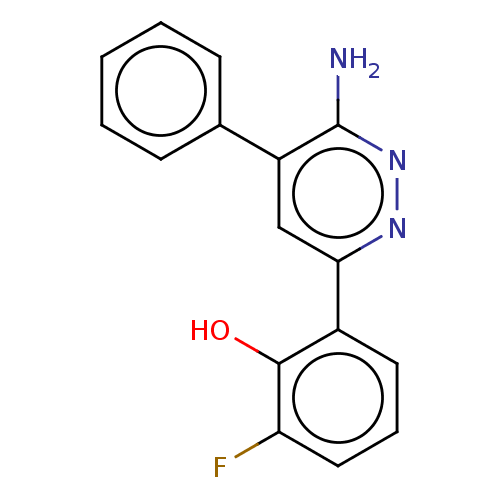 (2-(6-amino-5-phenylpyridazin-3-yl)-6-fluorophenol ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM394406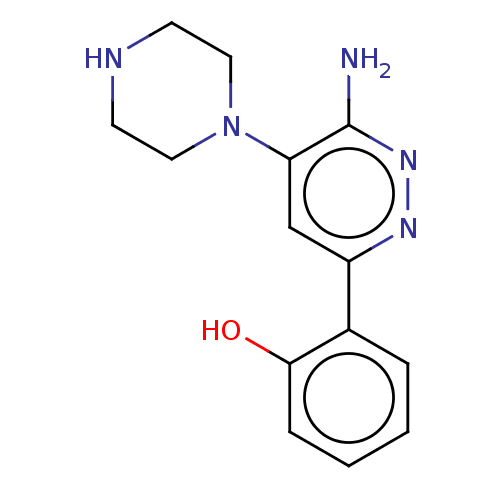 (2-(6-amino-5-(piperazin- | US10308614, Example 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Short of Probable global transcription activator SNF2L2 (Short) 377-1486] (Homo sapiens (Human)) | BDBM394613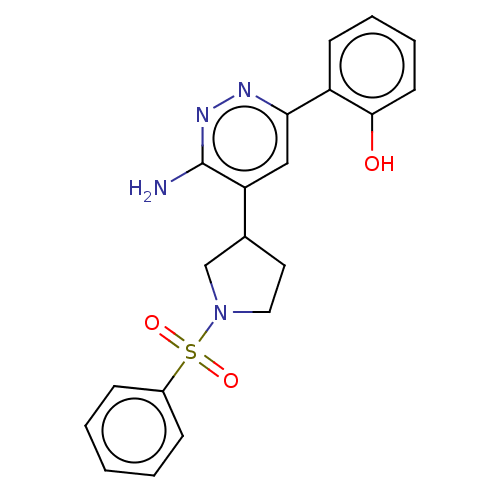 (2-(6-amino-5-(1-(phenylsulfonyl)pyrrolidin-3-yl)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 466 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research | Assay Description Histidine epitope tagged BRM (Isoform 2) Bromodomain1377-1486 (S1377-Q1486; Swiss Prot P51531-2; mhhhhhhgslvpr\gsSPNPPKLTKQMNAIIDTVINYKDSSGRQLSEVFIQL... | J Med Chem 52: 2255-64 (2009) BindingDB Entry DOI: 10.7270/Q26112NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM646010 ((2S,4R)-4-hydroxy-1-((2S)-2-(2-(2-(2- hydroxypheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM646012 ((2S,4R)-4-hydroxy-N-[[4-(5-methyl-1,3- thiazol-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable global transcription activator SNF2L2 (Homo sapiens (Human)) | BDBM50481805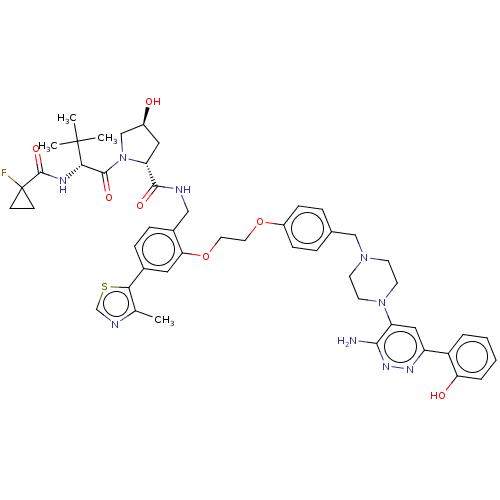 (CHEMBL5278697) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 63 total ) | Next | Last >> |