

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50444550 (CHEMBL3099704) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of rat recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50249047 (CHEMBL4080420) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat CYP11B2-1F4 expressed in Chinese hamster V79 cells using 11-deoxycorticosterone as substrate preincubated for 1 hr followed by subs... | Bioorg Med Chem Lett 27: 2384-2388 (2017) Article DOI: 10.1016/j.bmcl.2017.04.021 BindingDB Entry DOI: 10.7270/Q2MC92F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50249044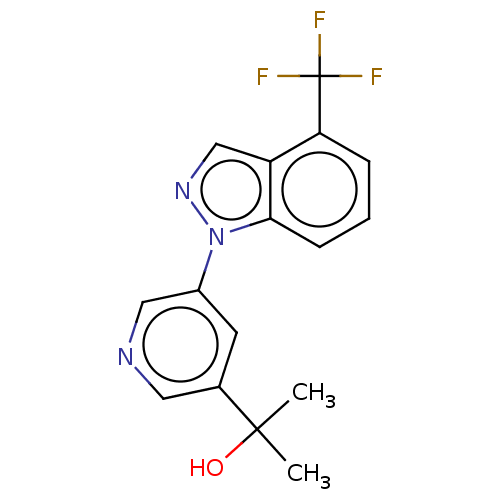 (CHEMBL4067000) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat CYP11B2-1F4 expressed in Chinese hamster V79 cells using 11-deoxycorticosterone as substrate preincubated for 1 hr followed by subs... | Bioorg Med Chem Lett 27: 2384-2388 (2017) Article DOI: 10.1016/j.bmcl.2017.04.021 BindingDB Entry DOI: 10.7270/Q2MC92F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50249045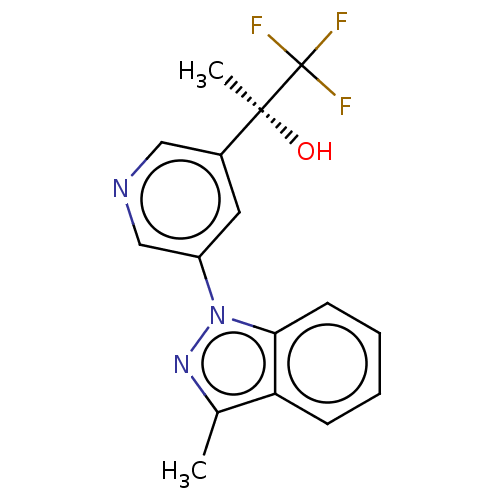 (CHEMBL4098297) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat CYP11B2-1F4 expressed in Chinese hamster V79 cells using 11-deoxycorticosterone as substrate preincubated for 1 hr followed by subs... | Bioorg Med Chem Lett 27: 2384-2388 (2017) Article DOI: 10.1016/j.bmcl.2017.04.021 BindingDB Entry DOI: 10.7270/Q2MC92F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50444548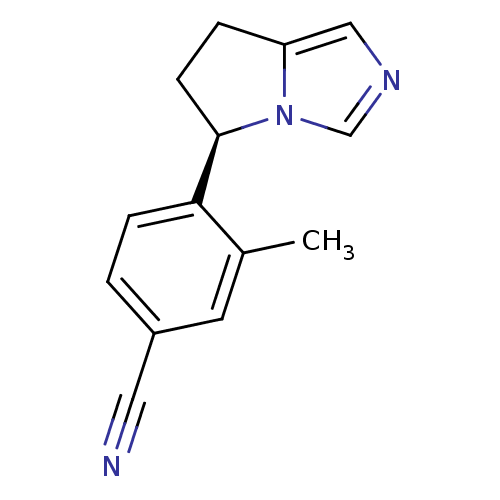 (CHEMBL3099696) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of rat recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50249048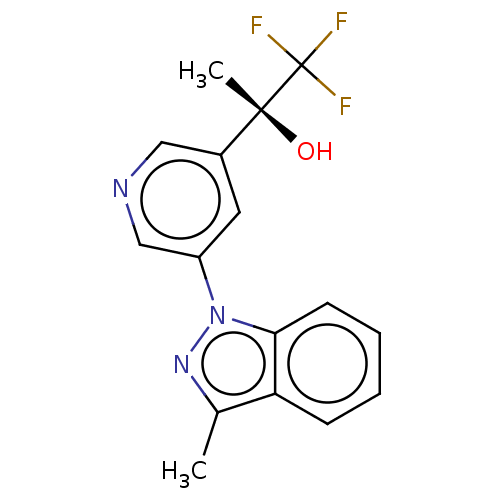 (CHEMBL4080256) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat CYP11B2-1F4 expressed in Chinese hamster V79 cells using 11-deoxycorticosterone as substrate preincubated for 1 hr followed by subs... | Bioorg Med Chem Lett 27: 2384-2388 (2017) Article DOI: 10.1016/j.bmcl.2017.04.021 BindingDB Entry DOI: 10.7270/Q2MC92F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50249046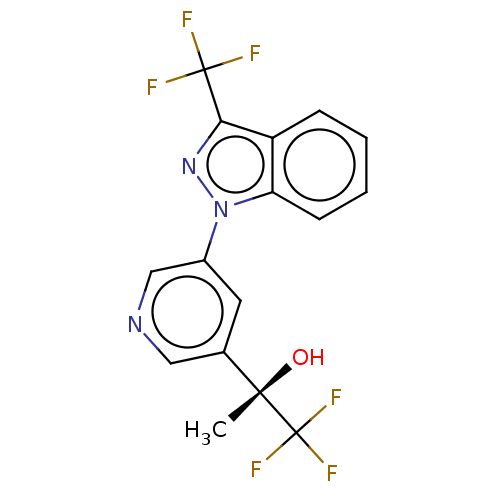 (CHEMBL4071689) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat CYP11B2-1F4 expressed in Chinese hamster V79 cells using 11-deoxycorticosterone as substrate preincubated for 1 hr followed by subs... | Bioorg Med Chem Lett 27: 2384-2388 (2017) Article DOI: 10.1016/j.bmcl.2017.04.021 BindingDB Entry DOI: 10.7270/Q2MC92F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50444549 (CHEMBL3099695) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of rat recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50047262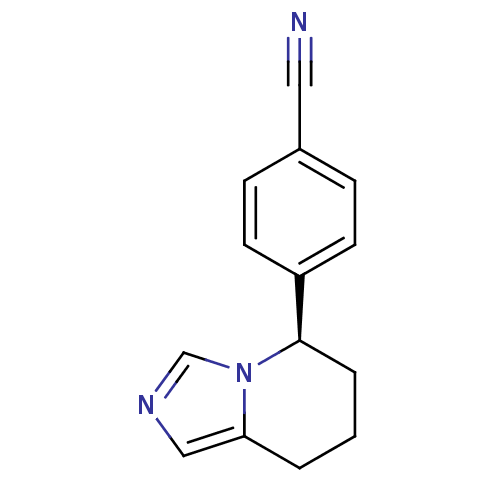 ((R)-4-(5,6,7,8-tetrahydroimidazo[1,5-a]pyridin-5-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of rat recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50238105 (CHEMBL4099824) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat CYP11B2 | Bioorg Med Chem Lett 27: 1902-1906 (2017) Article DOI: 10.1016/j.bmcl.2017.03.034 BindingDB Entry DOI: 10.7270/Q2KS6TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description In vitro inhibition of ACTH-stimulated aldosterone biosynthesis in rat adrenal slices | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50500189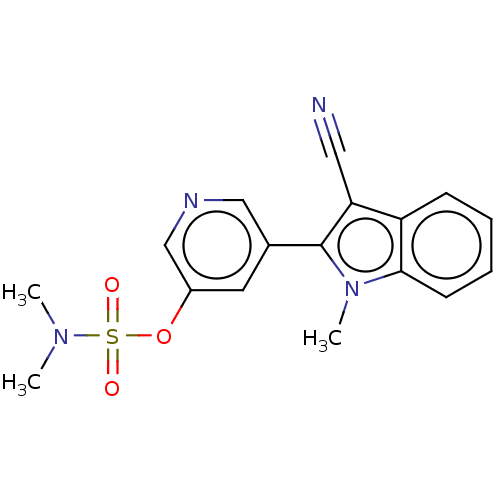 (CHEMBL3746175) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant rat CYP11B2 using 11-deoxycorticosterone as substrate after 2 hrs by scintillation proximity assay | J Med Chem 58: 9382-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b01545 BindingDB Entry DOI: 10.7270/Q2CC13QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50035209 (4-(1a,2,3,7b-Tetrahydro-1H-cyclopropa[a]naphthalen...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description In vitro inhibition of ACTH-stimulated aldosterone biosynthesis in rat adrenal slices | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50500185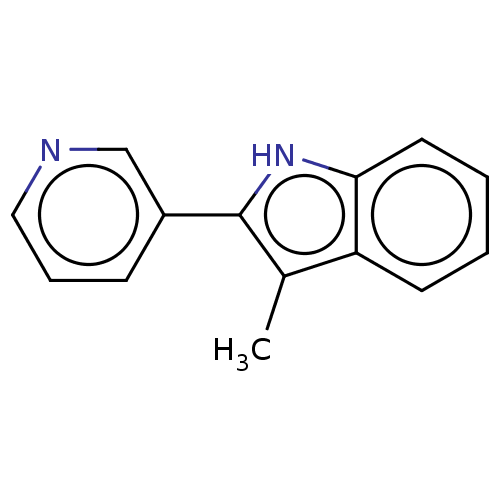 (CHEMBL3746125) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant rat CYP11B2 using 11-deoxycorticosterone as substrate after 2 hrs by scintillation proximity assay | J Med Chem 58: 9382-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b01545 BindingDB Entry DOI: 10.7270/Q2CC13QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50035208 (1-Pyridin-4-yl-1a,2,3,7b-tetrahydro-1H-cyclopropa[...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description In vitro inhibition of ACTH-stimulated aldosterone biosynthesis in rat adrenal slices | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50500187 (CHEMBL3747269) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant rat CYP11B2 using 11-deoxycorticosterone as substrate after 2 hrs by scintillation proximity assay | J Med Chem 58: 9382-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b01545 BindingDB Entry DOI: 10.7270/Q2CC13QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50035204 (4-(6-Methoxy-1a,2,3,7b-tetrahydro-1H-cyclopropa[a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description In vitro inhibition of ACTH-stimulated aldosterone biosynthesis in rat adrenal slices | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50035204 (4-(6-Methoxy-1a,2,3,7b-tetrahydro-1H-cyclopropa[a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description In vitro inhibition of ACTH-stimulated aldosterone biosynthesis in rat adrenal slices | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50500184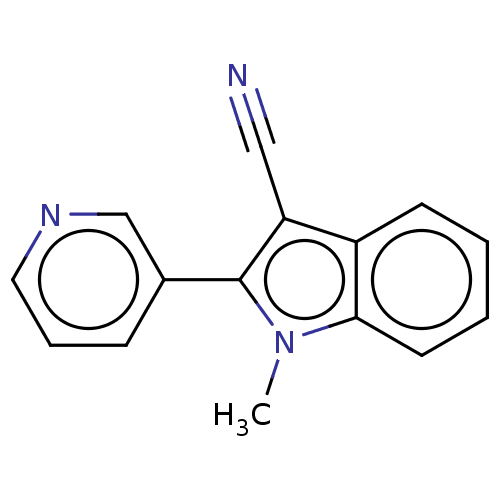 (CHEMBL3746868) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant rat CYP11B2 using 11-deoxycorticosterone as substrate after 2 hrs by scintillation proximity assay | J Med Chem 58: 9382-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b01545 BindingDB Entry DOI: 10.7270/Q2CC13QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50378780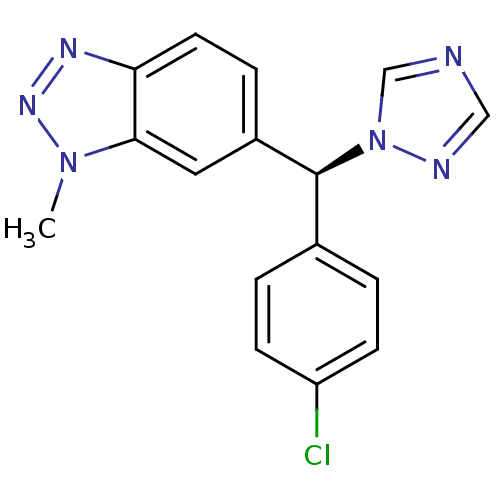 ((S)-VOROZOLE) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description In vitro inhibition of ACTH-stimulated aldosterone biosynthesis in rat adrenal slices | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50500188 (CHEMBL3747562) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant rat CYP11B2 using 11-deoxycorticosterone as substrate after 2 hrs by scintillation proximity assay | J Med Chem 58: 9382-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b01545 BindingDB Entry DOI: 10.7270/Q2CC13QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50011762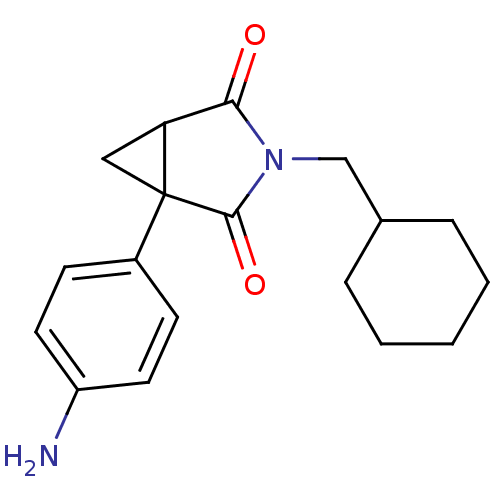 ((1R,5S)1-(4-Amino-phenyl)-3-cyclohexylmethyl-3-aza...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG. Curated by ChEMBL | Assay Description In vitro inhibition of aldosterone production in rat adrenal tissue | J Med Chem 34: 1329-34 (1991) BindingDB Entry DOI: 10.7270/Q2QN65RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description In vitro inhibition of ACTH-stimulated aldosterone biosynthesis in rat adrenal slices | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50500186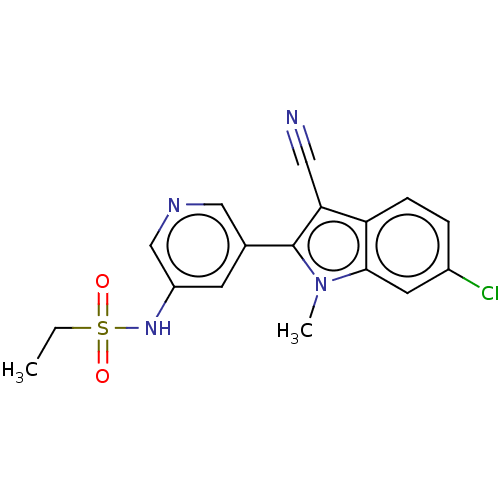 (CHEMBL3747069) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant rat CYP11B2 using 11-deoxycorticosterone as substrate after 2 hrs by scintillation proximity assay | J Med Chem 58: 9382-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b01545 BindingDB Entry DOI: 10.7270/Q2CC13QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität des Saarlandes Curated by ChEMBL | Assay Description In vitro inhibition of ACTH-stimulated aldosterone biosynthesis in rat adrenal slices | J Med Chem 38: 2103-11 (1995) BindingDB Entry DOI: 10.7270/Q2NV9H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG. Curated by ChEMBL | Assay Description In vitro inhibition of aldosterone production in rat adrenal tissue | J Med Chem 34: 1329-34 (1991) BindingDB Entry DOI: 10.7270/Q2QN65RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50011756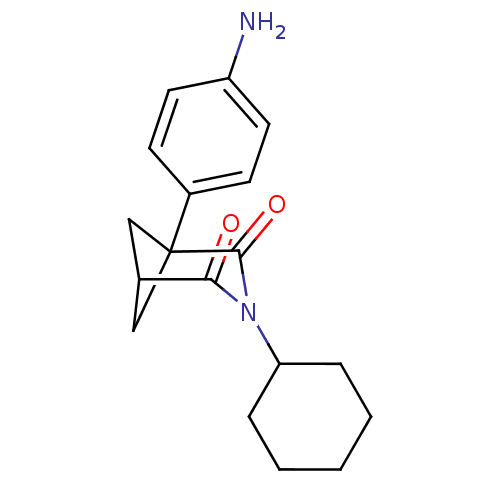 (1-(4-Amino-phenyl)-3-cyclohexyl-3-aza-bicyclo[3.1....) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG. Curated by ChEMBL | Assay Description In vitro inhibition of aldosterone production in rat adrenal tissue | J Med Chem 34: 1329-34 (1991) BindingDB Entry DOI: 10.7270/Q2QN65RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50011762 ((1R,5S)1-(4-Amino-phenyl)-3-cyclohexylmethyl-3-aza...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG. Curated by ChEMBL | Assay Description In vitro inhibition of estrogen production in hamster ovarian tissue | J Med Chem 34: 1329-34 (1991) BindingDB Entry DOI: 10.7270/Q2QN65RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||