 Found 82 hits of ic50 for UniProtKB: P41252
Found 82 hits of ic50 for UniProtKB: P41252 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50290686

(9-((E)-4-((2S,3R,4R,5S)-3,4-dihydroxy-5-(((2S,3S)-...)Show SMILES C[C@H](O)[C@H](C)[C@@H]1O[C@H]1C[C@H]1CO[C@@H](C\C(C)=C\C(=O)OCCCCCCCCC(O)=O)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C26H44O9/c1-16(13-23(30)33-11-9-7-5-4-6-8-10-22(28)29)12-20-25(32)24(31)19(15-34-20)14-21-26(35-21)17(2)18(3)27/h13,17-21,24-27,31-32H,4-12,14-15H2,1-3H3,(H,28,29)/b16-13+/t17-,18-,19-,20-,21-,24+,25-,26-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against Isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 7: 2805-2808 (1997)
Article DOI: 10.1016/S0960-894X(97)10088-9
BindingDB Entry DOI: 10.7270/Q21Z44FK |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50217841
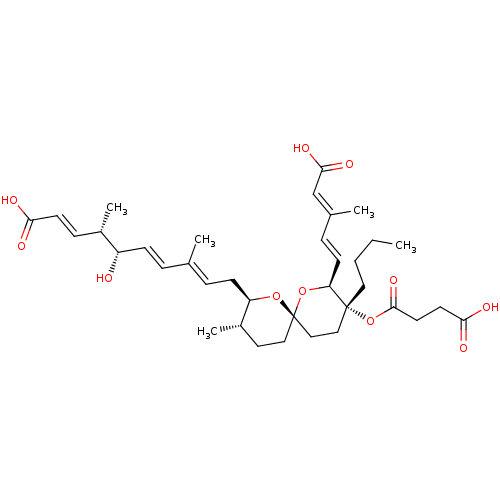
(REVEROMYCIN A)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](C)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C36H52O11/c1-6-7-19-35(47-34(44)17-16-32(40)41)21-22-36(46-30(35)14-10-25(3)23-33(42)43)20-18-27(5)29(45-36)13-9-24(2)8-12-28(37)26(4)11-15-31(38)39/h8-12,14-15,23,26-30,37H,6-7,13,16-22H2,1-5H3,(H,38,39)(H,40,41)(H,42,43)/b12-8+,14-10+,15-11+,24-9+,25-23+/t26-,27-,28-,29+,30-,35+,36-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.46 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50366870
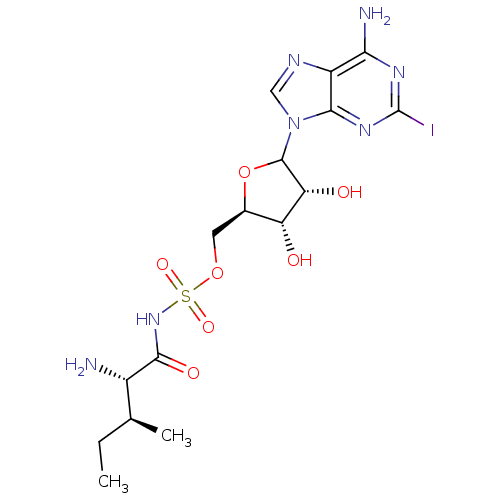
(CHEMBL605376)Show SMILES CC[C@H](C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)nc(I)nc12 |r| Show InChI InChI=1S/C16H24IN7O7S/c1-3-6(2)8(18)14(27)23-32(28,29)30-4-7-10(25)11(26)15(31-7)24-5-20-9-12(19)21-16(17)22-13(9)24/h5-8,10-11,15,25-26H,3-4,18H2,1-2H3,(H,23,27)(H2,19,21,22)/t6-,7+,8-,10+,11+,15?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli Isoleucyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 13: 1087-92 (2003)
BindingDB Entry DOI: 10.7270/Q29K4BRS |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075058

(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1cccc(OC)c1 Show InChI InChI=1S/C21H29N3O8S2/c1-4-11(2)16(22)20(27)24-34(28,29)31-9-15-17(25)18(26)19(32-15)21-23-14(10-33-21)12-6-5-7-13(8-12)30-3/h5-8,10-11,15-19,25-26H,4,9,22H2,1-3H3,(H,24,27)/t11?,15-,16+,17-,18-,19-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of human isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479281
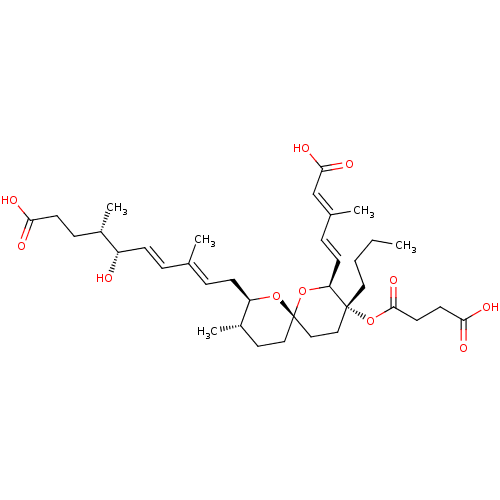
(2,3-DIHYDROREVEROMYCIN A)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](C)CCC(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C36H54O11/c1-6-7-19-35(47-34(44)17-16-32(40)41)21-22-36(46-30(35)14-10-25(3)23-33(42)43)20-18-27(5)29(45-36)13-9-24(2)8-12-28(37)26(4)11-15-31(38)39/h8-10,12,14,23,26-30,37H,6-7,11,13,15-22H2,1-5H3,(H,38,39)(H,40,41)(H,42,43)/b12-8+,14-10+,24-9+,25-23+/t26-,27-,28-,29+,30-,35+,36-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17.5 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075061
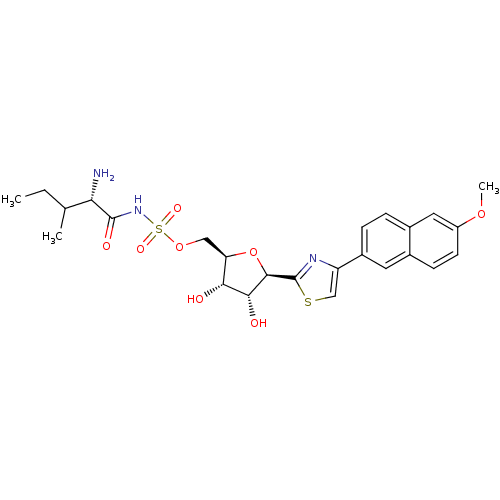
(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1ccc2cc(OC)ccc2c1 Show InChI InChI=1S/C25H31N3O8S2/c1-4-13(2)20(26)24(31)28-38(32,33)35-11-19-21(29)22(30)23(36-19)25-27-18(12-37-25)16-6-5-15-10-17(34-3)8-7-14(15)9-16/h5-10,12-13,19-23,29-30H,4,11,26H2,1-3H3,(H,28,31)/t13?,19-,20+,21-,22-,23-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of E. coli isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479274

(4-HYDROXY REVEROMYCIN A)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](O)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C35H50O12/c1-5-6-18-34(47-33(44)16-15-31(40)41)20-21-35(46-29(34)13-9-24(3)22-32(42)43)19-17-25(4)28(45-35)12-8-23(2)7-10-26(36)27(37)11-14-30(38)39/h7-11,13-14,22,25-29,36-37H,5-6,12,15-21H2,1-4H3,(H,38,39)(H,40,41)(H,42,43)/b10-7+,13-9+,14-11+,23-8+,24-22+/t25-,26-,27-,28+,29-,34+,35-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21.7 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075059

(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1ccccc1OC Show InChI InChI=1S/C21H29N3O8S2/c1-4-11(2)16(22)20(27)24-34(28,29)31-9-15-17(25)18(26)19(32-15)21-23-13(10-33-21)12-7-5-6-8-14(12)30-3/h5-8,10-11,15-19,25-26H,4,9,22H2,1-3H3,(H,24,27)/t11?,15-,16+,17-,18-,19-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of E. coli isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075056

(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(CCc2ccccc2)cs1 Show InChI InChI=1S/C22H31N3O7S2/c1-3-13(2)17(23)21(28)25-34(29,30)31-11-16-18(26)19(27)20(32-16)22-24-15(12-33-22)10-9-14-7-5-4-6-8-14/h4-8,12-13,16-20,26-27H,3,9-11,23H2,1-2H3,(H,25,28)/t13?,16-,17+,18-,19-,20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of E. coli isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075058

(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1cccc(OC)c1 Show InChI InChI=1S/C21H29N3O8S2/c1-4-11(2)16(22)20(27)24-34(28,29)31-9-15-17(25)18(26)19(32-15)21-23-14(10-33-21)12-6-5-7-13(8-12)30-3/h5-8,10-11,15-19,25-26H,4,9,22H2,1-3H3,(H,24,27)/t11?,15-,16+,17-,18-,19-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of human isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479284

(CHEMBL442945)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](C)CO)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C34H52O10/c1-6-7-17-33(44-32(41)15-14-30(37)38)19-20-34(43-29(33)13-10-24(3)21-31(39)40)18-16-25(4)28(42-34)12-9-23(2)8-11-27(36)26(5)22-35/h8-11,13,21,25-29,35-36H,6-7,12,14-20,22H2,1-5H3,(H,37,38)(H,39,40)/b11-8+,13-10+,23-9+,24-21+/t25-,26-,27-,28+,29-,33+,34-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36.9 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50366862

(CHEMBL605592)Show SMILES CC[C@H](C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)nc(nc12)C#C |r| Show InChI InChI=1S/C18H25N7O7S/c1-4-8(3)11(19)17(28)24-33(29,30)31-6-9-13(26)14(27)18(32-9)25-7-21-12-15(20)22-10(5-2)23-16(12)25/h2,7-9,11,13-14,18,26-27H,4,6,19H2,1,3H3,(H,24,28)(H2,20,22,23)/t8-,9+,11-,13+,14+,18?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli Isoleucyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 13: 1087-92 (2003)
BindingDB Entry DOI: 10.7270/Q29K4BRS |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075056

(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(CCc2ccccc2)cs1 Show InChI InChI=1S/C22H31N3O7S2/c1-3-13(2)17(23)21(28)25-34(29,30)31-11-16-18(26)19(27)20(32-16)22-24-15(12-33-22)10-9-14-7-5-4-6-8-14/h4-8,12-13,16-20,26-27H,3,9-11,23H2,1-2H3,(H,25,28)/t13?,16-,17+,18-,19-,20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of S. aureus isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075070
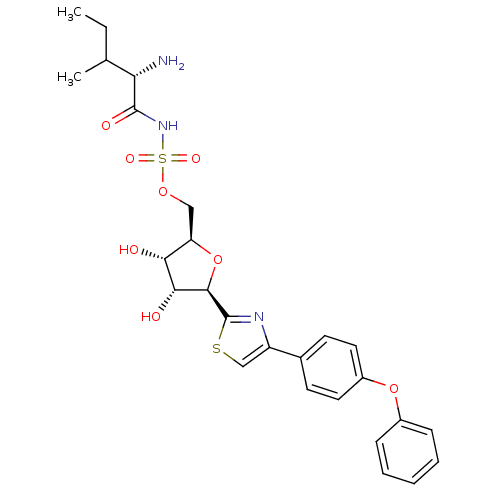
(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C26H31N3O8S2/c1-3-15(2)21(27)25(32)29-39(33,34)35-13-20-22(30)23(31)24(37-20)26-28-19(14-38-26)16-9-11-18(12-10-16)36-17-7-5-4-6-8-17/h4-12,14-15,20-24,30-31H,3,13,27H2,1-2H3,(H,29,32)/t15?,20-,21+,22-,23-,24-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of human Leucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479277

(2,3-DIHYDRO-5-EPIREVEROMYCIN A)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@@H](O)[C@@H](C)CCC(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C36H54O11/c1-6-7-19-35(47-34(44)17-16-32(40)41)21-22-36(46-30(35)14-10-25(3)23-33(42)43)20-18-27(5)29(45-36)13-9-24(2)8-12-28(37)26(4)11-15-31(38)39/h8-10,12,14,23,26-30,37H,6-7,11,13,15-22H2,1-5H3,(H,38,39)(H,40,41)(H,42,43)/b12-8+,14-10+,24-9+,25-23+/t26-,27-,28+,29+,30-,35+,36-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50222902

(CHEMBL1163069)Show SMILES CC[C@H](C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C16H25N7O7S/c1-3-7(2)9(17)15(26)22-31(27,28)29-4-8-11(24)12(25)16(30-8)23-6-21-10-13(18)19-5-20-14(10)23/h5-9,11-12,16,24-25H,3-4,17H2,1-2H3,(H,22,26)(H2,18,19,20)/t7-,8+,9-,11+,12+,16+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli Isoleucyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 13: 1087-92 (2003)
BindingDB Entry DOI: 10.7270/Q29K4BRS |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075063

(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1ccc(OC)cc1 Show InChI InChI=1S/C21H29N3O8S2/c1-4-11(2)16(22)20(27)24-34(28,29)31-9-15-17(25)18(26)19(32-15)21-23-14(10-33-21)12-5-7-13(30-3)8-6-12/h5-8,10-11,15-19,25-26H,4,9,22H2,1-3H3,(H,24,27)/t11?,15-,16+,17-,18-,19-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of E. coli isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075068

(((2S,3S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid...)Show SMILES CC[C@H](C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C20H27N3O7S2/c1-3-11(2)15(21)19(26)23-32(27,28)29-9-14-16(24)17(25)18(30-14)20-22-13(10-31-20)12-7-5-4-6-8-12/h4-8,10-11,14-18,24-25H,3,9,21H2,1-2H3,(H,23,26)/t11-,14+,15-,16+,17+,18+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of E. coli isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50217935
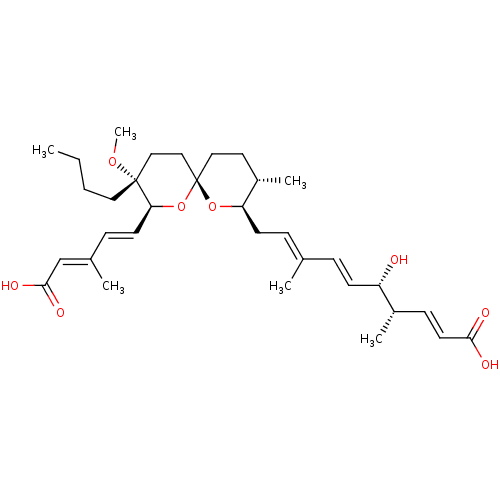
(CHEMBL114859)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](C)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C33H50O8/c1-7-8-18-32(39-6)20-21-33(41-29(32)15-11-24(3)22-31(37)38)19-17-26(5)28(40-33)14-10-23(2)9-13-27(34)25(4)12-16-30(35)36/h9-13,15-16,22,25-29,34H,7-8,14,17-21H2,1-6H3,(H,35,36)(H,37,38)/b13-9+,15-11+,16-12+,23-10+,24-22+/t25-,26-,27-,28+,29-,32+,33-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50290689
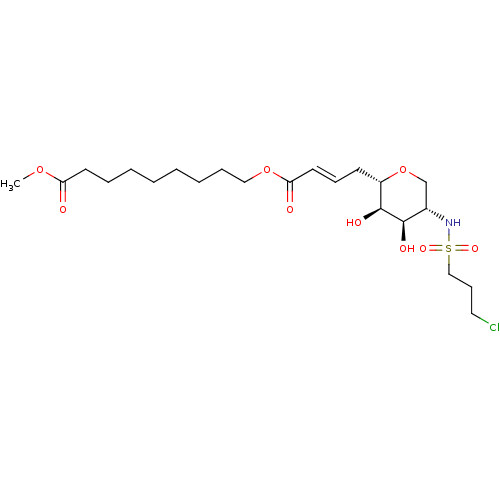
(9-{(E)-4-[(2S,3R,4R,5S)-5-(3-Chloro-propane-1-sulf...)Show SMILES COC(=O)CCCCCCCCOC(=O)\C=C\C[C@@H]1OC[C@H](NS(=O)(=O)CCCCl)[C@@H](O)[C@H]1O Show InChI InChI=1S/C22H38ClNO9S/c1-31-19(25)11-6-4-2-3-5-7-14-32-20(26)12-8-10-18-22(28)21(27)17(16-33-18)24-34(29,30)15-9-13-23/h8,12,17-18,21-22,24,27-28H,2-7,9-11,13-16H2,1H3/b12-8+/t17-,18-,21+,22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against Isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 7: 2805-2808 (1997)
Article DOI: 10.1016/S0960-894X(97)10088-9
BindingDB Entry DOI: 10.7270/Q21Z44FK |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075065
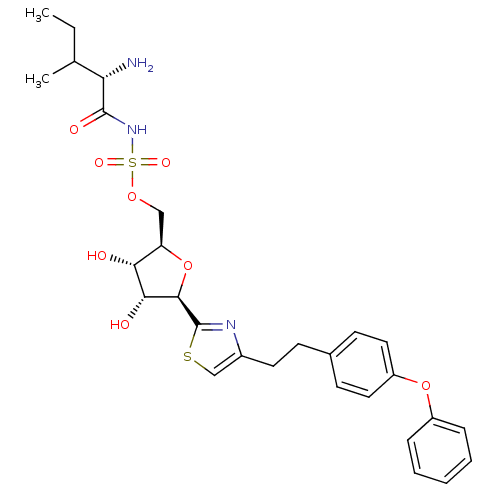
(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(CCc2ccc(Oc3ccccc3)cc2)cs1 Show InChI InChI=1S/C28H35N3O8S2/c1-3-17(2)23(29)27(34)31-41(35,36)37-15-22-24(32)25(33)26(39-22)28-30-19(16-40-28)12-9-18-10-13-21(14-11-18)38-20-7-5-4-6-8-20/h4-8,10-11,13-14,16-17,22-26,32-33H,3,9,12,15,29H2,1-2H3,(H,31,34)/t17?,22-,23+,24-,25-,26-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of E. coli isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075062
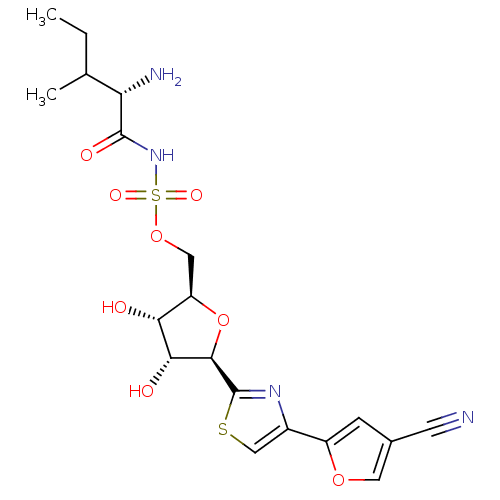
(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1cc(co1)C#N Show InChI InChI=1S/C19H24N4O8S2/c1-3-9(2)14(21)18(26)23-33(27,28)30-7-13-15(24)16(25)17(31-13)19-22-11(8-32-19)12-4-10(5-20)6-29-12/h4,6,8-9,13-17,24-25H,3,7,21H2,1-2H3,(H,23,26)/t9?,13-,14+,15-,16-,17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of E. coli isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50217844
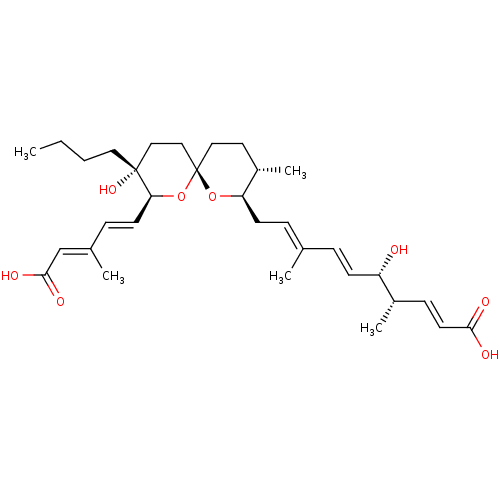
(CHEMBL115953)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](C)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@](O)(CCCC)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C32H48O8/c1-6-7-17-31(38)19-20-32(40-28(31)14-10-23(3)21-30(36)37)18-16-25(5)27(39-32)13-9-22(2)8-12-26(33)24(4)11-15-29(34)35/h8-12,14-15,21,24-28,33,38H,6-7,13,16-20H2,1-5H3,(H,34,35)(H,36,37)/b12-8+,14-10+,15-11+,22-9+,23-21+/t24-,25-,26-,27+,28-,31+,32-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 168 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075063

(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1ccc(OC)cc1 Show InChI InChI=1S/C21H29N3O8S2/c1-4-11(2)16(22)20(27)24-34(28,29)31-9-15-17(25)18(26)19(32-15)21-23-14(10-33-21)12-5-7-13(30-3)8-6-12/h5-8,10-11,15-19,25-26H,4,9,22H2,1-3H3,(H,24,27)/t11?,15-,16+,17-,18-,19-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of S. aureus Leucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075062

(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1cc(co1)C#N Show InChI InChI=1S/C19H24N4O8S2/c1-3-9(2)14(21)18(26)23-33(27,28)30-7-13-15(24)16(25)17(31-13)19-22-11(8-32-19)12-4-10(5-20)6-29-12/h4,6,8-9,13-17,24-25H,3,7,21H2,1-2H3,(H,23,26)/t9?,13-,14+,15-,16-,17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of S. aureus isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075059

(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1ccccc1OC Show InChI InChI=1S/C21H29N3O8S2/c1-4-11(2)16(22)20(27)24-34(28,29)31-9-15-17(25)18(26)19(32-15)21-23-13(10-33-21)12-7-5-6-8-14(12)30-3/h5-8,10-11,15-19,25-26H,4,9,22H2,1-3H3,(H,24,27)/t11?,15-,16+,17-,18-,19-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of S. aureus isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50290688

(9-{(E)-4-[(2S,3R,4R,5S)-5-(3-Chloro-benzenesulfony...)Show SMILES COC(=O)CCCCCCCCOC(=O)\C=C\C[C@@H]1OC[C@H](NS(=O)(=O)c2cccc(Cl)c2)[C@@H](O)[C@H]1O Show InChI InChI=1S/C25H36ClNO9S/c1-34-22(28)13-6-4-2-3-5-7-15-35-23(29)14-9-12-21-25(31)24(30)20(17-36-21)27-37(32,33)19-11-8-10-18(26)16-19/h8-11,14,16,20-21,24-25,27,30-31H,2-7,12-13,15,17H2,1H3/b14-9+/t20-,21-,24+,25-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against Isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 7: 2805-2808 (1997)
Article DOI: 10.1016/S0960-894X(97)10088-9
BindingDB Entry DOI: 10.7270/Q21Z44FK |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50290687
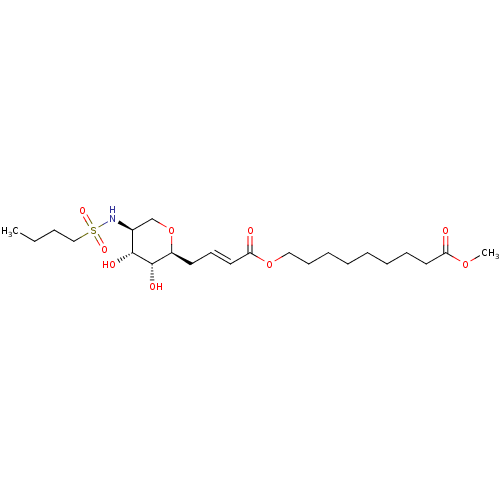
(9-{(E)-4-[(2S,3R,4R,5S)-5-(Butane-1-sulfonylamino)...)Show SMILES CCCCS(=O)(=O)N[C@H]1CO[C@@H](C\C=C\C(=O)OCCCCCCCCC(=O)OC)[C@H](O)[C@@H]1O Show InChI InChI=1S/C23H41NO9S/c1-3-4-16-34(29,30)24-18-17-33-19(23(28)22(18)27)12-11-14-21(26)32-15-10-8-6-5-7-9-13-20(25)31-2/h11,14,18-19,22-24,27-28H,3-10,12-13,15-17H2,1-2H3/b14-11+/t18-,19-,22+,23-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against Isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 7: 2805-2808 (1997)
Article DOI: 10.1016/S0960-894X(97)10088-9
BindingDB Entry DOI: 10.7270/Q21Z44FK |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479282

(CHEMBL505238)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](C)\C=C\C(=O)OC)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C37H54O11/c1-7-8-20-36(48-35(44)18-16-32(39)40)22-23-37(47-31(36)15-11-26(3)24-33(41)42)21-19-28(5)30(46-37)14-10-25(2)9-13-29(38)27(4)12-17-34(43)45-6/h9-13,15,17,24,27-31,38H,7-8,14,16,18-23H2,1-6H3,(H,39,40)(H,41,42)/b13-9+,15-11+,17-12+,25-10+,26-24+/t27-,28-,29-,30+,31-,36+,37-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 312 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075068

(((2S,3S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid...)Show SMILES CC[C@H](C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C20H27N3O7S2/c1-3-11(2)15(21)19(26)23-32(27,28)29-9-14-16(24)17(25)18(30-14)20-22-13(10-31-20)12-7-5-4-6-8-12/h4-8,10-11,14-18,24-25H,3,9,21H2,1-2H3,(H,23,26)/t11-,14+,15-,16+,17+,18+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of human isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075070

(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C26H31N3O8S2/c1-3-15(2)21(27)25(32)29-39(33,34)35-13-20-22(30)23(31)24(37-20)26-28-19(14-38-26)16-9-11-18(12-10-16)36-17-7-5-4-6-8-17/h4-12,14-15,20-24,30-31H,3,13,27H2,1-2H3,(H,29,32)/t15?,20-,21+,22-,23-,24-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of human isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075061

(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(cs1)-c1ccc2cc(OC)ccc2c1 Show InChI InChI=1S/C25H31N3O8S2/c1-4-13(2)20(26)24(31)28-38(32,33)35-11-19-21(29)22(30)23(36-19)25-27-18(12-37-25)16-6-5-15-10-17(34-3)8-7-14(15)9-16/h5-10,12-13,19-23,29-30H,4,11,26H2,1-3H3,(H,28,31)/t13?,19-,20+,21-,22-,23-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of human isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479276
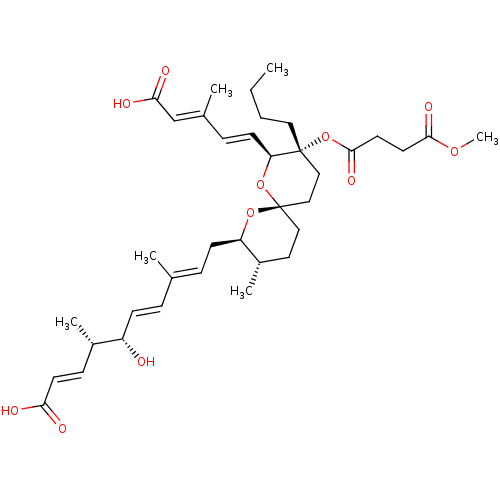
(CHEMBL461144)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](C)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(=O)OC)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C37H54O11/c1-7-8-20-36(48-35(44)18-17-34(43)45-6)22-23-37(47-31(36)15-11-26(3)24-33(41)42)21-19-28(5)30(46-37)14-10-25(2)9-13-29(38)27(4)12-16-32(39)40/h9-13,15-16,24,27-31,38H,7-8,14,17-23H2,1-6H3,(H,39,40)(H,41,42)/b13-9+,15-11+,16-12+,25-10+,26-24+/t27-,28-,29-,30+,31-,36+,37-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 433 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50217849
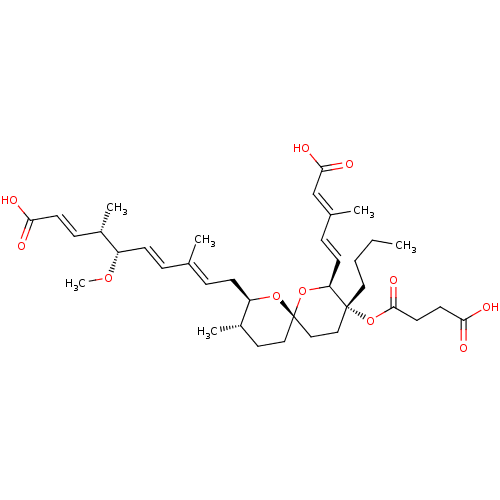
(CHEMBL115317)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](OC)[C@@H](C)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C37H54O11/c1-7-8-20-36(48-35(44)18-17-33(40)41)22-23-37(47-31(36)15-11-26(3)24-34(42)43)21-19-28(5)30(46-37)14-10-25(2)9-13-29(45-6)27(4)12-16-32(38)39/h9-13,15-16,24,27-31H,7-8,14,17-23H2,1-6H3,(H,38,39)(H,40,41)(H,42,43)/b13-9+,15-11+,16-12+,25-10+,26-24+/t27-,28-,29-,30+,31-,36+,37-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 554 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479275
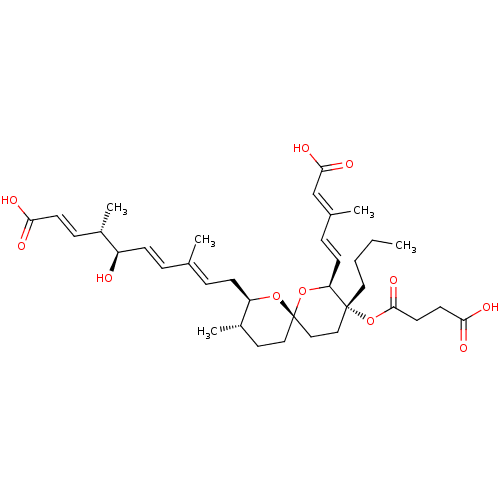
(5-EPIREVEROMYCIN A)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@@H](O)[C@@H](C)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C36H52O11/c1-6-7-19-35(47-34(44)17-16-32(40)41)21-22-36(46-30(35)14-10-25(3)23-33(42)43)20-18-27(5)29(45-36)13-9-24(2)8-12-28(37)26(4)11-15-31(38)39/h8-12,14-15,23,26-30,37H,6-7,13,16-22H2,1-5H3,(H,38,39)(H,40,41)(H,42,43)/b12-8+,14-10+,15-11+,24-9+,25-23+/t26-,27-,28+,29+,30-,35+,36-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 572 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50217839
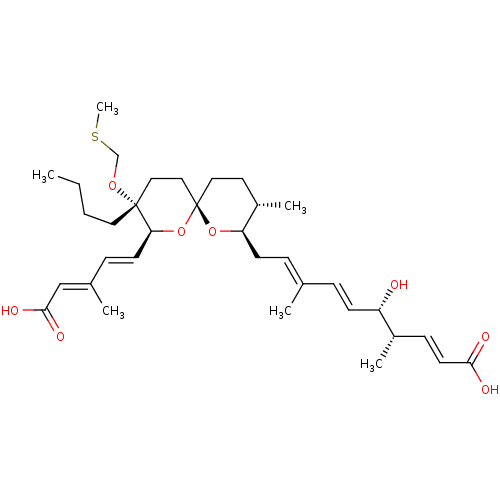
(CHEMBL327131)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](C)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OCSC)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C34H52O8S/c1-7-8-18-33(40-23-43-6)20-21-34(42-30(33)15-11-25(3)22-32(38)39)19-17-27(5)29(41-34)14-10-24(2)9-13-28(35)26(4)12-16-31(36)37/h9-13,15-16,22,26-30,35H,7-8,14,17-21,23H2,1-6H3,(H,36,37)(H,38,39)/b13-9+,15-11+,16-12+,24-10+,25-22+/t26-,27-,28-,29+,30-,33+,34-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 801 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479273

(CHEMBL498825)Show SMILES [H][C@]1(C\C=C(/C)\C=C\CO)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C31H46O9/c1-5-6-16-30(40-29(37)14-13-27(33)34)18-19-31(39-26(30)12-10-23(3)21-28(35)36)17-15-24(4)25(38-31)11-9-22(2)8-7-20-32/h7-10,12,21,24-26,32H,5-6,11,13-20H2,1-4H3,(H,33,34)(H,35,36)/b8-7+,12-10+,22-9+,23-21+/t24-,25+,26-,30+,31-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 995 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50075065

(((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(CCc2ccc(Oc3ccccc3)cc2)cs1 Show InChI InChI=1S/C28H35N3O8S2/c1-3-17(2)23(29)27(34)31-41(35,36)37-15-22-24(32)25(33)26(39-22)28-30-19(16-40-28)12-9-18-10-13-21(14-11-18)38-20-7-5-4-6-8-20/h4-8,10-11,13-14,16-17,22-26,32-33H,3,9,12,15,29H2,1-2H3,(H,31,34)/t17?,22-,23+,24-,25-,26-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound tested for the inhibition of human isoleucyl-tRNA synthetase |
Bioorg Med Chem Lett 9: 375-80 (1999)
BindingDB Entry DOI: 10.7270/Q2M32TX1 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50366874
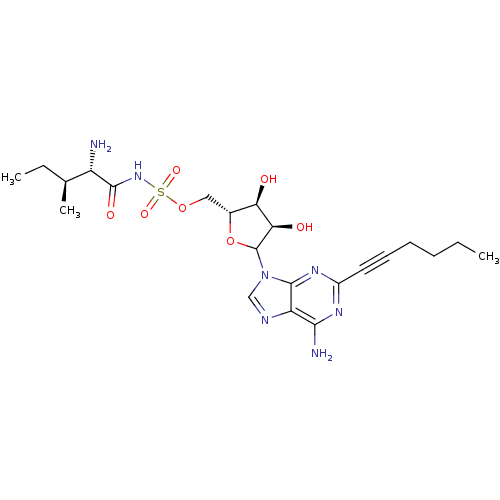
(CHEMBL605809)Show SMILES CCCCC#Cc1nc(N)c2ncn(C3O[C@H](COS(=O)(=O)NC(=O)[C@@H](N)[C@@H](C)CC)[C@@H](O)[C@H]3O)c2n1 |r| Show InChI InChI=1S/C22H33N7O7S/c1-4-6-7-8-9-14-26-19(24)16-20(27-14)29(11-25-16)22-18(31)17(30)13(36-22)10-35-37(33,34)28-21(32)15(23)12(3)5-2/h11-13,15,17-18,22,30-31H,4-7,10,23H2,1-3H3,(H,28,32)(H2,24,26,27)/t12-,13+,15-,17+,18+,22?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli Isoleucyl-tRNA synthetase was determined |
Bioorg Med Chem Lett 13: 1087-92 (2003)
BindingDB Entry DOI: 10.7270/Q29K4BRS |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479280
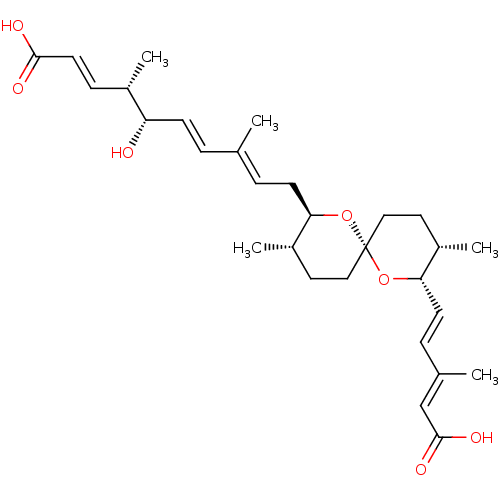
(SPIROFUNGIN A)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](C)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@H](C)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C29H42O7/c1-19(6-10-24(30)21(3)9-13-27(31)32)7-11-25-22(4)14-16-29(35-25)17-15-23(5)26(36-29)12-8-20(2)18-28(33)34/h6-10,12-13,18,21-26,30H,11,14-17H2,1-5H3,(H,31,32)(H,33,34)/b10-6+,12-8+,13-9+,19-7+,20-18+/t21-,22-,23-,24-,25+,26-,29-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479272

(CHEMBL455272)Show SMILES [H][C@]1([#6]\[#6]=[#6](/[#6])\[#6]=[#6]\[#6@H](-[#8][Si;v4]([#6])([#6])C([#6])([#6])[#6])-[#6@@H](-[#6])\[#6]=[#6]\[#6](-[#8])=O)[#8][C@]2([#6]-[#6]-[#6@@H]1-[#6])[#6]-[#6][C@@]([#6]-[#6]-[#6]-[#6])([#8]-[#6](=O)-[#6]-[#6]-[#6](-[#8])=O)[C@@]([H])([#8]2)\[#6]=[#6]\[#6](\[#6])=[#6]\[#6](-[#8])=O |r| Show InChI InChI=1S/C42H66O11Si/c1-11-12-24-41(52-39(49)22-21-37(45)46)26-27-42(51-35(41)19-15-30(3)28-38(47)48)25-23-32(5)33(50-42)17-13-29(2)14-18-34(31(4)16-20-36(43)44)53-54(9,10)40(6,7)8/h13-16,18-20,28,31-35H,11-12,17,21-27H2,1-10H3,(H,43,44)(H,45,46)(H,47,48)/b18-14+,19-15+,20-16+,29-13+,30-28+/t31-,32-,33+,34-,35-,41+,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50217937
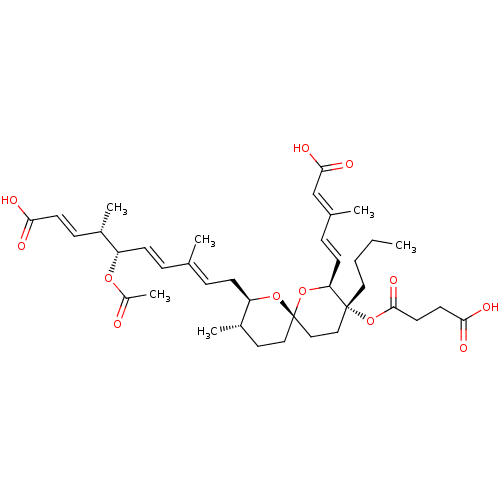
(CHEMBL114949)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](OC(C)=O)[C@@H](C)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C38H54O12/c1-7-8-20-37(50-36(46)18-17-34(42)43)22-23-38(49-32(37)15-11-26(3)24-35(44)45)21-19-28(5)31(48-38)14-10-25(2)9-13-30(47-29(6)39)27(4)12-16-33(40)41/h9-13,15-16,24,27-28,30-32H,7-8,14,17-23H2,1-6H3,(H,40,41)(H,42,43)(H,44,45)/b13-9+,15-11+,16-12+,25-10+,26-24+/t27-,28-,30-,31+,32-,37+,38-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479279

(CHEMBL510665)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](C)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(=O)OC |r| Show InChI InChI=1S/C37H54O11/c1-7-8-20-36(48-34(43)18-17-33(41)42)22-23-37(47-31(36)15-11-26(3)24-35(44)45-6)21-19-28(5)30(46-37)14-10-25(2)9-13-29(38)27(4)12-16-32(39)40/h9-13,15-16,24,27-31,38H,7-8,14,17-23H2,1-6H3,(H,39,40)(H,41,42)/b13-9+,15-11+,16-12+,25-10+,26-24+/t27-,28-,29-,30+,31-,36+,37-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50217842
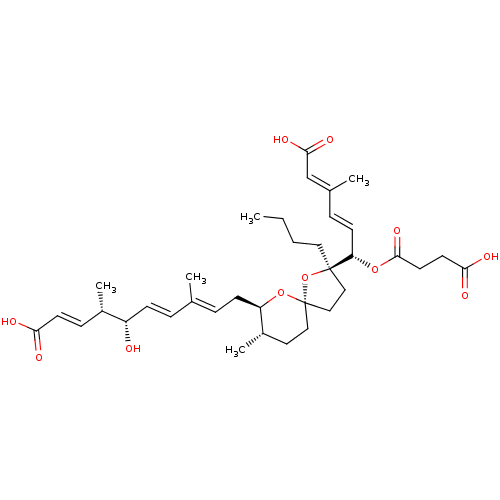
(REVEROMYCIN B)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](C)\C=C\C(O)=O)O[C@@]2(CC[C@@](CCCC)(O2)[C@@H](OC(=O)CCC(O)=O)\C=C\C(\C)=C\C(O)=O)CC[C@@H]1C |r| Show InChI InChI=1S/C36H52O11/c1-6-7-19-35(30(14-10-25(3)23-33(42)43)45-34(44)17-16-32(40)41)21-22-36(47-35)20-18-27(5)29(46-36)13-9-24(2)8-12-28(37)26(4)11-15-31(38)39/h8-12,14-15,23,26-30,37H,6-7,13,16-22H2,1-5H3,(H,38,39)(H,40,41)(H,42,43)/b12-8+,14-10+,15-11+,24-9+,25-23+/t26-,27-,28-,29+,30-,35+,36-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479270
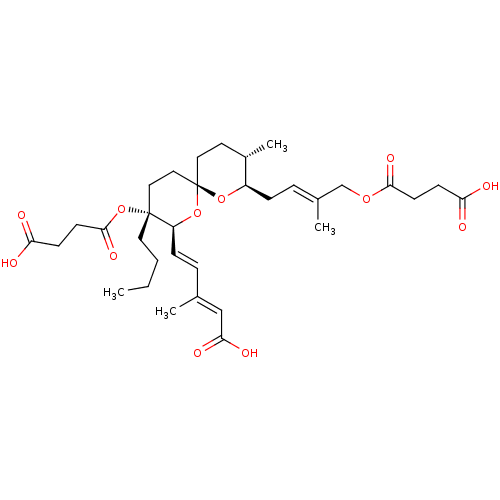
(CHEMBL499655)Show SMILES [H][C@]1(C\C=C(/C)COC(=O)CCC(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C33H48O12/c1-5-6-16-32(45-31(41)14-12-28(36)37)18-19-33(44-26(32)10-8-22(2)20-29(38)39)17-15-24(4)25(43-33)9-7-23(3)21-42-30(40)13-11-27(34)35/h7-8,10,20,24-26H,5-6,9,11-19,21H2,1-4H3,(H,34,35)(H,36,37)(H,38,39)/b10-8+,22-20+,23-7+/t24-,25+,26-,32+,33-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479283
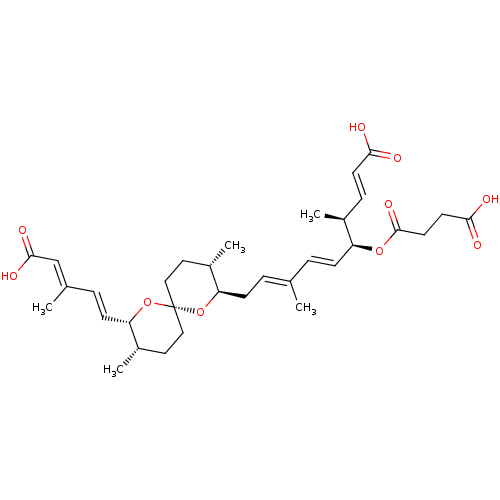
(CHEMBL508146)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](OC(=O)CCC(O)=O)[C@@H](C)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@H](C)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C33H46O10/c1-21(6-10-26(23(3)9-13-29(34)35)41-32(40)15-14-30(36)37)7-11-27-24(4)16-18-33(42-27)19-17-25(5)28(43-33)12-8-22(2)20-31(38)39/h6-10,12-13,20,23-28H,11,14-19H2,1-5H3,(H,34,35)(H,36,37)(H,38,39)/b10-6+,12-8+,13-9+,21-7+,22-20+/t23-,24-,25-,26-,27+,28-,33-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479269

(CHEMBL471857)Show SMILES [H][C@]1(C\C=C(/C)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C31H44O10/c1-5-6-16-30(41-29(38)14-13-27(34)35)18-19-31(40-25(30)11-8-22(3)20-28(36)37)17-15-23(4)24(39-31)10-7-21(2)9-12-26(32)33/h7-9,11-12,20,23-25H,5-6,10,13-19H2,1-4H3,(H,32,33)(H,34,35)(H,36,37)/b11-8+,12-9+,21-7+,22-20+/t23-,24+,25-,30+,31-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479271

(CHEMBL515541)Show SMILES [H][C@]1(C\C=C(/C)CO)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C29H44O9/c1-5-6-14-28(38-27(35)12-11-25(31)32)16-17-29(37-24(28)10-8-20(2)18-26(33)34)15-13-22(4)23(36-29)9-7-21(3)19-30/h7-8,10,18,22-24,30H,5-6,9,11-17,19H2,1-4H3,(H,31,32)(H,33,34)/b10-8+,20-18+,21-7+/t22-,23+,24-,28+,29-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479268
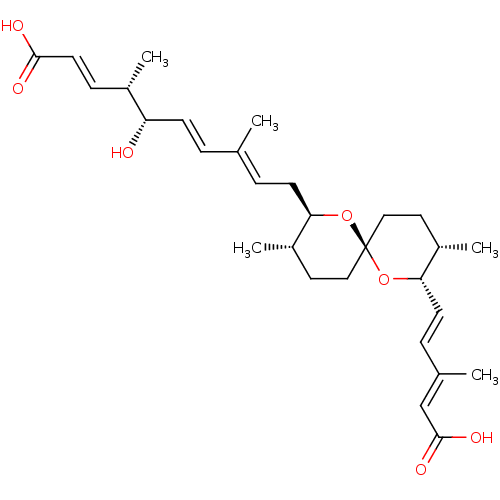
(SPIROFUNGIN B)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](C)\C=C\C(O)=O)O[C@@]2(CC[C@@H]1C)CC[C@H](C)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C29H42O7/c1-19(6-10-24(30)21(3)9-13-27(31)32)7-11-25-22(4)14-16-29(35-25)17-15-23(5)26(36-29)12-8-20(2)18-28(33)34/h6-10,12-13,18,21-26,30H,11,14-17H2,1-5H3,(H,31,32)(H,33,34)/b10-6+,12-8+,13-9+,19-7+,20-18+/t21-,22-,23-,24-,25+,26-,29+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479278

(CHEMBL462123)Show SMILES [H][C@]1(CCO)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C26H40O9/c1-4-5-12-25(35-24(32)9-8-22(28)29)14-15-26(13-10-19(3)20(33-26)11-16-27)34-21(25)7-6-18(2)17-23(30)31/h6-7,17,19-21,27H,4-5,8-16H2,1-3H3,(H,28,29)(H,30,31)/b7-6+,18-17+/t19-,20+,21-,25+,26-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data