 Found 55 hits of ic50 for UniProtKB: Q02880
Found 55 hits of ic50 for UniProtKB: Q02880 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50449890

(CHEMBL4161876)Show SMILES COc1cc(cc(OC)c1OC)C1=N\C(=C/c2cc(Br)cc(Br)c2OC(C)=O)C(=O)NN1c1ncc(s1)-c1ccccc1 |t:13| Show InChI InChI=1S/C30H24Br2N4O6S/c1-16(37)42-26-18(10-20(31)14-21(26)32)11-22-29(38)35-36(30-33-15-25(43-30)17-8-6-5-7-9-17)28(34-22)19-12-23(39-2)27(41-4)24(13-19)40-3/h5-15H,1-4H3,(H,35,38)/b22-11- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Port Said University
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 2beta after 2 hrs by ELISA |
Eur J Med Chem 156: 563-579 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.003
BindingDB Entry DOI: 10.7270/Q20G3NPD |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50449891

(CHEMBL4174756)Show SMILES COc1cc(cc(OC)c1OC)C1=N\C(=C/c2cc(Br)cc(Br)c2OC(C)=O)C(=O)NN1c1ccccc1 |t:13| Show InChI InChI=1S/C27H23Br2N3O6/c1-15(33)38-24-16(10-18(28)14-20(24)29)11-21-27(34)31-32(19-8-6-5-7-9-19)26(30-21)17-12-22(35-2)25(37-4)23(13-17)36-3/h5-14H,1-4H3,(H,31,34)/b21-11- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Port Said University
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 2beta after 2 hrs by ELISA |
Eur J Med Chem 156: 563-579 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.003
BindingDB Entry DOI: 10.7270/Q20G3NPD |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50410828

(CHEMBL5266387)Show SMILES [#7]-[#6](=O)-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)[C@@]([#7])([#6]-[#8])[#6]-[#16]-[#16]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C47H67N15O13S2/c48-35(65)15-14-29-39(69)59-32(20-36(49)66)42(72)60-33(44(74)62-17-5-9-34(62)43(73)57-28(8-4-16-54-46(51)52)38(68)55-21-37(50)67)22-76-77-24-47(53,23-63)45(75)61-31(19-26-10-12-27(64)13-11-26)41(71)58-30(40(70)56-29)18-25-6-2-1-3-7-25/h1-3,6-7,10-13,28-34,63-64H,4-5,8-9,14-24,53H2,(H2,48,65)(H2,49,66)(H2,50,67)(H,55,68)(H,56,70)(H,57,73)(H,58,71)(H,59,69)(H,60,72)(H,61,75)(H4,51,52,54)/t28-,29-,30-,31-,32-,33+,34-,47+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity in phenoxybenzamine-treated rat by Pressor assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50520631

(Ametantrone)Show SMILES OCCNCCNc1ccc(NCCNCCO)c2C(=O)c3ccccc3C(=O)c12 Show InChI InChI=1S/C22H28N4O4/c27-13-11-23-7-9-25-17-5-6-18(26-10-8-24-12-14-28)20-19(17)21(29)15-3-1-2-4-16(15)22(20)30/h1-6,23-28H,7-14H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Poison activity at recombinant human topoisomerase 2beta using pBR322 plasmid as substrate after 30 mins by ethidium bromide staining based agarose g... |
J Med Chem 61: 8947-8980 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01202
BindingDB Entry DOI: 10.7270/Q2H998M5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50449889

(CHEMBL4160802)Show SMILES COc1cc(cc(OC)c1OC)C1=N\C(=C/c2cc(Br)cc(Br)c2OC(C)=O)C(=O)NN1C(N)=S |t:13| Show InChI InChI=1S/C22H20Br2N4O6S/c1-10(29)34-18-11(5-13(23)9-14(18)24)6-15-21(30)27-28(22(25)35)20(26-15)12-7-16(31-2)19(33-4)17(8-12)32-3/h5-9H,1-4H3,(H2,25,35)(H,27,30)/b15-6- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Port Said University
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 2beta after 2 hrs by ELISA |
Eur J Med Chem 156: 563-579 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.003
BindingDB Entry DOI: 10.7270/Q20G3NPD |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50127140

((-)-etoposide | (5S,5aR,8aR,9R)-9-(4-hydroxy-3,5-d...)Show SMILES COc1cc(cc(OC)c1O)[C@H]1[C@@H]2[C@H](COC2=O)[C@H](O[C@@H]2O[C@@H]3CO[C@@H](C)O[C@H]3[C@H](O)[C@H]2O)c2cc3OCOc3cc12 |r| Show InChI InChI=1S/C29H32O13/c1-11-36-9-20-27(40-11)24(31)25(32)29(41-20)42-26-14-7-17-16(38-10-39-17)6-13(14)21(22-15(26)8-37-28(22)33)12-4-18(34-2)23(30)19(5-12)35-3/h4-7,11,15,20-22,24-27,29-32H,8-10H2,1-3H3/t11-,15+,20-,21-,22+,24-,25-,26-,27-,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Port Said University
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 2beta after 2 hrs by ELISA |
Eur J Med Chem 156: 563-579 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.003
BindingDB Entry DOI: 10.7270/Q20G3NPD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50449888

(CHEMBL4172196)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N\C(=C\c1cc(Br)cc(Br)c1OC(C)=O)C(=O)NNC(N)=S Show InChI InChI=1S/C22H22Br2N4O7S/c1-10(29)35-18-11(5-13(23)9-14(18)24)6-15(21(31)27-28-22(25)36)26-20(30)12-7-16(32-2)19(34-4)17(8-12)33-3/h5-9H,1-4H3,(H,26,30)(H,27,31)(H3,25,28,36)/b15-6+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Port Said University
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 2beta after 2 hrs by ELISA |
Eur J Med Chem 156: 563-579 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.003
BindingDB Entry DOI: 10.7270/Q20G3NPD |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50449887

(CHEMBL4169404)Show SMILES COc1cc(cc(OC)c1OC)C1=N\C(=C/c2cc(Br)cc(Br)c2OC(C)=O)C(=O)n2c(C)nc(=S)n12 |t:13| Show InChI InChI=1S/C24H20Br2N4O6S/c1-11-27-24(37)30-22(14-8-18(33-3)21(35-5)19(9-14)34-4)28-17(23(32)29(11)30)7-13-6-15(25)10-16(26)20(13)36-12(2)31/h6-10H,1-5H3/b17-7- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Port Said University
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 2beta after 2 hrs by ELISA |
Eur J Med Chem 156: 563-579 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.003
BindingDB Entry DOI: 10.7270/Q20G3NPD |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50547613
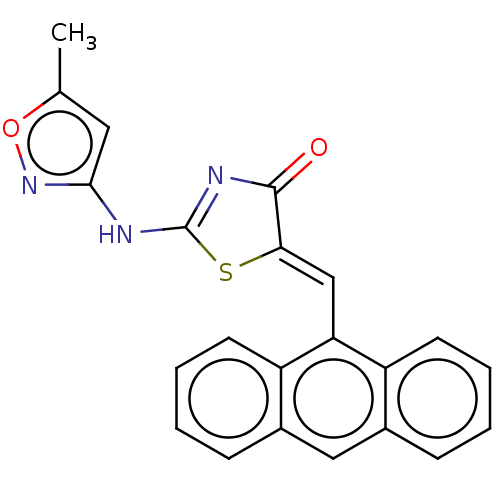
(CHEMBL4751362)Show SMILES Cc1cc(NC2=NC(=O)\C(S2)=C/c2c3ccccc3cc3ccccc23)no1 |t:5| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Topoisomerase II beta (unknown origin) relative to control |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115674
BindingDB Entry DOI: 10.7270/Q2TM7FQQ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50473498

(CHEMBL157831 | Ro-616653)Show SMILES COC(=O)c1c(Br)c(OC)cc(O)c1CSC[C@H](Nc1nc(cs1)-c1ccc(cc1)[N+]([O-])=O)c1nc(C)no1 |r| Show InChI InChI=1S/C24H22BrN5O7S2/c1-12-26-22(37-29-12)17(28-24-27-16(11-39-24)13-4-6-14(7-5-13)30(33)34)10-38-9-15-18(31)8-19(35-2)21(25)20(15)23(32)36-3/h4-8,11,17,31H,9-10H2,1-3H3,(H,27,28)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 157 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antibacterial activity against Micrococcus luteus DNA gyrase. |
J Med Chem 44: 619-26 (2001)
Article DOI: 10.1021/jm0010623
BindingDB Entry DOI: 10.7270/Q2T43WT3 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50473498

(CHEMBL157831 | Ro-616653)Show SMILES COC(=O)c1c(Br)c(OC)cc(O)c1CSC[C@H](Nc1nc(cs1)-c1ccc(cc1)[N+]([O-])=O)c1nc(C)no1 |r| Show InChI InChI=1S/C24H22BrN5O7S2/c1-12-26-22(37-29-12)17(28-24-27-16(11-39-24)13-4-6-14(7-5-13)30(33)34)10-38-9-15-18(31)8-19(35-2)21(25)20(15)23(32)36-3/h4-8,11,17,31H,9-10H2,1-3H3,(H,27,28)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 157 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antibacterial activity against Escherichia coli DNA gyrase |
J Med Chem 44: 619-26 (2001)
Article DOI: 10.1021/jm0010623
BindingDB Entry DOI: 10.7270/Q2T43WT3 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50473499

(BAY-507950 | CHEMBL156813)Show SMILES COC(=O)[C@H](CSCc1c(O)cc(OC)c(Br)c1C(=O)OC)Nc1nc(cs1)-c1ccc(cc1)[N+]([O-])=O |r| Show InChI InChI=1S/C23H22BrN3O8S2/c1-33-18-8-17(28)14(19(20(18)24)22(30)35-3)9-36-10-16(21(29)34-2)26-23-25-15(11-37-23)12-4-6-13(7-5-12)27(31)32/h4-8,11,16,28H,9-10H2,1-3H3,(H,25,26)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 326 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antibacterial activity against Micrococcus luteus DNA gyrase. |
J Med Chem 44: 619-26 (2001)
Article DOI: 10.1021/jm0010623
BindingDB Entry DOI: 10.7270/Q2T43WT3 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50229974

(CHEMBL178077)Show SMILES OC(=O)c1c2scc3COc4c(N5CCNCC5)c(F)cc(c4n23)c1=O Show InChI InChI=1S/C17H14FN3O4S/c18-10-5-9-12-15(13(10)20-3-1-19-2-4-20)25-6-8-7-26-16(21(8)12)11(14(9)22)17(23)24/h5,7,19H,1-4,6H2,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 452 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
The inhibitory activity was measured for the supercoiling activity of DNA gyrase isolated from Escherichia coli K-12 C600. |
J Med Chem 35: 94-9 (1992)
BindingDB Entry DOI: 10.7270/Q2D79DNN |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50473499

(BAY-507950 | CHEMBL156813)Show SMILES COC(=O)[C@H](CSCc1c(O)cc(OC)c(Br)c1C(=O)OC)Nc1nc(cs1)-c1ccc(cc1)[N+]([O-])=O |r| Show InChI InChI=1S/C23H22BrN3O8S2/c1-33-18-8-17(28)14(19(20(18)24)22(30)35-3)9-36-10-16(21(29)34-2)26-23-25-15(11-37-23)12-4-6-13(7-5-12)27(31)32/h4-8,11,16,28H,9-10H2,1-3H3,(H,25,26)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 489 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antibacterial activity against Escherichia coli DNA gyrase |
J Med Chem 44: 619-26 (2001)
Article DOI: 10.1021/jm0010623
BindingDB Entry DOI: 10.7270/Q2T43WT3 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50458474
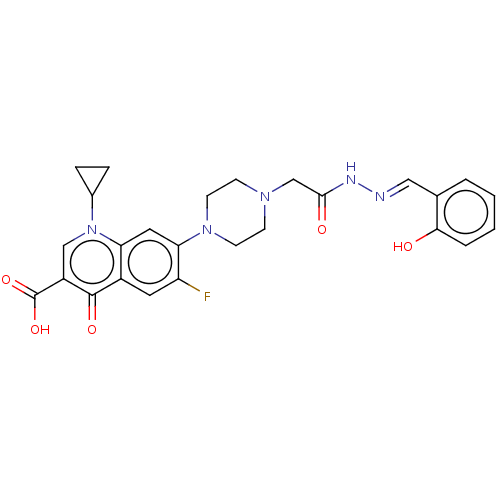
(CHEMBL4213218)Show SMILES OC(=O)c1cn(C2CC2)c2cc(N3CCN(CC(=O)N\N=C\c4ccccc4O)CC3)c(F)cc2c1=O Show InChI InChI=1S/C26H26FN5O5/c27-20-11-18-21(32(17-5-6-17)14-19(25(18)35)26(36)37)12-22(20)31-9-7-30(8-10-31)15-24(34)29-28-13-16-3-1-2-4-23(16)33/h1-4,11-14,17,33H,5-10,15H2,(H,29,34)(H,36,37)/b28-13+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of human topoisomerase-2B after 2 hrs by ELISA |
Eur J Med Chem 150: 403-418 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.026
BindingDB Entry DOI: 10.7270/Q2MS3WCZ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM21690

(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...)Show InChI InChI=1S/C17H18FN3O3/c18-13-7-11-14(8-15(13)20-5-3-19-4-6-20)21(10-1-2-10)9-12(16(11)22)17(23)24/h7-10,19H,1-6H2,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 603 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
The inhibitory activity was measured for the supercoiling activity of DNA gyrase isolated from Escherichia coli K-12 C600. |
J Med Chem 35: 94-9 (1992)
BindingDB Entry DOI: 10.7270/Q2D79DNN |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50473497
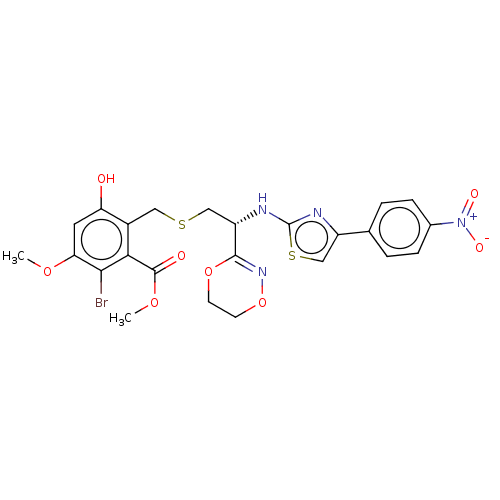
(BAY-507952 | CHEMBL157769)Show SMILES COC(=O)c1c(Br)c(OC)cc(O)c1CSC[C@H](Nc1nc(cs1)-c1ccc(cc1)[N+]([O-])=O)C1=NOCCO1 |r,t:36| Show InChI InChI=1S/C24H23BrN4O8S2/c1-34-19-9-18(30)15(20(21(19)25)23(31)35-2)10-38-11-17(22-28-37-8-7-36-22)27-24-26-16(12-39-24)13-3-5-14(6-4-13)29(32)33/h3-6,9,12,17,30H,7-8,10-11H2,1-2H3,(H,26,27)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 625 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antibacterial activity against Micrococcus luteus DNA gyrase. |
J Med Chem 44: 619-26 (2001)
Article DOI: 10.1021/jm0010623
BindingDB Entry DOI: 10.7270/Q2T43WT3 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50229967

(CHEMBL263944)Show SMILES NC1CCN(C1)c1c(F)cc2c3c1OCc1csc(c(C(O)=O)c2=O)n31 Show InChI InChI=1S/C17H14FN3O4S/c18-10-3-9-12-15(13(10)20-2-1-7(19)4-20)25-5-8-6-26-16(21(8)12)11(14(9)22)17(23)24/h3,6-7H,1-2,4-5,19H2,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 666 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
The inhibitory activity was measured for the supercoiling activity of DNA gyrase isolated from Escherichia coli K-12 C600. |
J Med Chem 35: 94-9 (1992)
BindingDB Entry DOI: 10.7270/Q2D79DNN |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM22984

((8S,10S)-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-me...)Show SMILES COc1cccc2C(=O)c3c(O)c4C[C@](O)(C[C@H](O[C@H]5C[C@H](N)[C@H](O)[C@H](C)O5)c4c(O)c3C(=O)c12)C(=O)CO |r| Show InChI InChI=1S/C27H29NO11/c1-10-22(31)13(28)6-17(38-10)39-15-8-27(36,16(30)9-29)7-12-19(15)26(35)21-20(24(12)33)23(32)11-4-3-5-14(37-2)18(11)25(21)34/h3-5,10,13,15,17,22,29,31,33,35-36H,6-9,28H2,1-2H3/t10-,13-,15-,17-,22+,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 727 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Topoisomerase II beta (unknown origin) relative to control |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115674
BindingDB Entry DOI: 10.7270/Q2TM7FQQ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50229969

(CHEMBL554645)Show SMILES Cl.CN1CCN(CC1)c1c(F)cc2c3c1OCc1csc(c(C(O)=O)c2=O)n31 Show InChI InChI=1S/C18H16FN3O4S/c1-20-2-4-21(5-3-20)14-11(19)6-10-13-16(14)26-7-9-8-27-17(22(9)13)12(15(10)23)18(24)25/h6,8H,2-5,7H2,1H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 727 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
The inhibitory activity was measured for the supercoiling activity of DNA gyrase isolated from Escherichia coli K-12 C600. |
J Med Chem 35: 94-9 (1992)
BindingDB Entry DOI: 10.7270/Q2D79DNN |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50473497

(BAY-507952 | CHEMBL157769)Show SMILES COC(=O)c1c(Br)c(OC)cc(O)c1CSC[C@H](Nc1nc(cs1)-c1ccc(cc1)[N+]([O-])=O)C1=NOCCO1 |r,t:36| Show InChI InChI=1S/C24H23BrN4O8S2/c1-34-19-9-18(30)15(20(21(19)25)23(31)35-2)10-38-11-17(22-28-37-8-7-36-22)27-24-26-16(12-39-24)13-3-5-14(6-4-13)29(32)33/h3-6,9,12,17,30H,7-8,10-11H2,1-2H3,(H,26,27)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 781 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antibacterial activity against Escherichia coli DNA gyrase |
J Med Chem 44: 619-26 (2001)
Article DOI: 10.1021/jm0010623
BindingDB Entry DOI: 10.7270/Q2T43WT3 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50127140

((-)-etoposide | (5S,5aR,8aR,9R)-9-(4-hydroxy-3,5-d...)Show SMILES COc1cc(cc(OC)c1O)[C@H]1[C@@H]2[C@H](COC2=O)[C@H](O[C@@H]2O[C@@H]3CO[C@@H](C)O[C@H]3[C@H](O)[C@H]2O)c2cc3OCOc3cc12 |r| Show InChI InChI=1S/C29H32O13/c1-11-36-9-20-27(40-11)24(31)25(32)29(41-20)42-26-14-7-17-16(38-10-39-17)6-13(14)21(22-15(26)8-37-28(22)33)12-4-18(34-2)23(30)19(5-12)35-3/h4-7,11,15,20-22,24-27,29-32H,8-10H2,1-3H3/t11-,15+,20-,21-,22+,24-,25-,26-,27-,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of human topoisomerase-2B after 2 hrs by ELISA |
Eur J Med Chem 150: 403-418 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.026
BindingDB Entry DOI: 10.7270/Q2MS3WCZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50229970
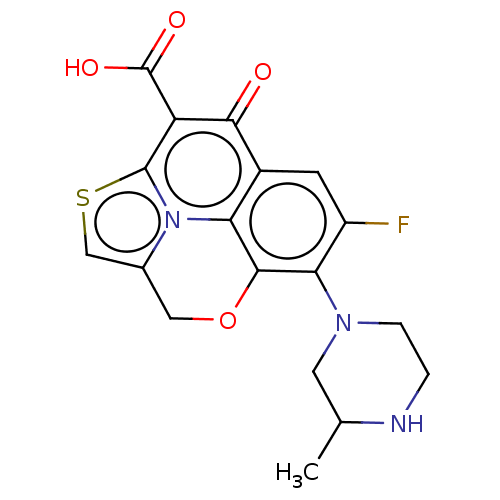
(CHEMBL367876)Show SMILES CC1CN(CCN1)c1c(F)cc2c3c1OCc1csc(c(C(O)=O)c2=O)n31 Show InChI InChI=1S/C18H16FN3O4S/c1-8-5-21(3-2-20-8)14-11(19)4-10-13-16(14)26-6-9-7-27-17(22(9)13)12(15(10)23)18(24)25/h4,7-8,20H,2-3,5-6H2,1H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 796 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
The inhibitory activity was measured for the supercoiling activity of DNA gyrase isolated from Escherichia coli K-12 C600. |
J Med Chem 35: 94-9 (1992)
BindingDB Entry DOI: 10.7270/Q2D79DNN |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50458475
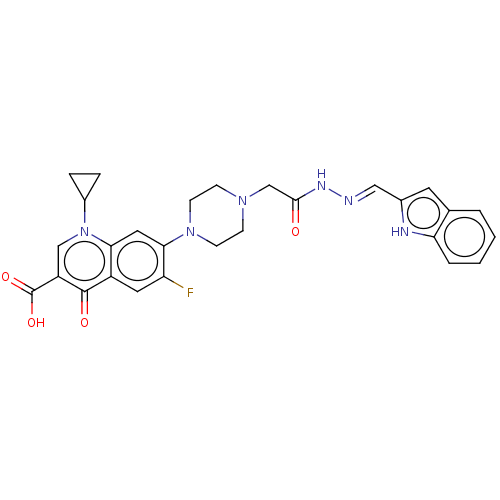
(CHEMBL4205327)Show SMILES OC(=O)c1cn(C2CC2)c2cc(N3CCN(CC(=O)N\N=C\c4cc5ccccc5[nH]4)CC3)c(F)cc2c1=O Show InChI InChI=1S/C28H27FN6O4/c29-22-12-20-24(35(19-5-6-19)15-21(27(20)37)28(38)39)13-25(22)34-9-7-33(8-10-34)16-26(36)32-30-14-18-11-17-3-1-2-4-23(17)31-18/h1-4,11-15,19,31H,5-10,16H2,(H,32,36)(H,38,39)/b30-14+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of human topoisomerase-2B after 2 hrs by ELISA |
Eur J Med Chem 150: 403-418 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.026
BindingDB Entry DOI: 10.7270/Q2MS3WCZ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM21690

(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...)Show InChI InChI=1S/C17H18FN3O3/c18-13-7-11-14(8-15(13)20-5-3-19-4-6-20)21(10-1-2-10)9-12(16(11)22)17(23)24/h7-10,19H,1-6H2,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 905 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling-Winthrop Research Institute
Curated by ChEMBL
| Assay Description
Gyrase inhibitory activity against Escherichia coli |
J Med Chem 31: 1694-7 (1988)
BindingDB Entry DOI: 10.7270/Q2RX9F84 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50229972
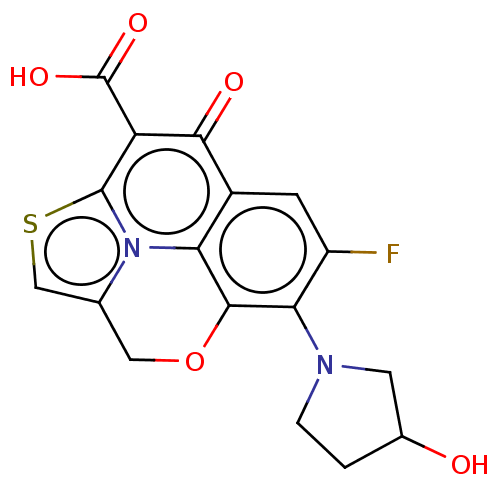
(CHEMBL174721)Show SMILES OC1CCN(C1)c1c(F)cc2c3c1OCc1csc(c(C(O)=O)c2=O)n31 Show InChI InChI=1S/C17H13FN2O5S/c18-10-3-9-12-15(13(10)19-2-1-8(21)4-19)25-5-7-6-26-16(20(7)12)11(14(9)22)17(23)24/h3,6,8,21H,1-2,4-5H2,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 929 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
The inhibitory activity was measured for the supercoiling activity of DNA gyrase isolated from Escherichia coli K-12 C600. |
J Med Chem 35: 94-9 (1992)
BindingDB Entry DOI: 10.7270/Q2D79DNN |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50520632
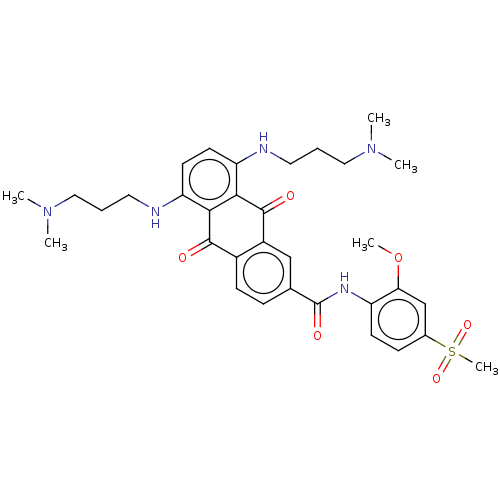
(CHEMBL4553471)Show SMILES COc1cc(ccc1NC(=O)c1ccc2C(=O)c3c(NCCCN(C)C)ccc(NCCCN(C)C)c3C(=O)c2c1)S(C)(=O)=O Show InChI InChI=1S/C33H41N5O6S/c1-37(2)17-7-15-34-26-13-14-27(35-16-8-18-38(3)4)30-29(26)31(39)23-11-9-21(19-24(23)32(30)40)33(41)36-25-12-10-22(45(6,42)43)20-28(25)44-5/h9-14,19-20,34-35H,7-8,15-18H2,1-6H3,(H,36,41) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Poison activity at recombinant human topoisomerase 2beta using pBR322 plasmid as substrate after 30 mins by ethidium bromide staining based agarose g... |
J Med Chem 61: 8947-8980 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01202
BindingDB Entry DOI: 10.7270/Q2H998M5 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50520634
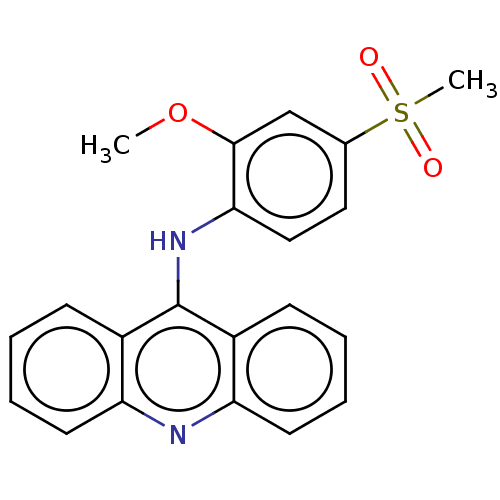
(CHEMBL4575796)Show SMILES COc1cc(ccc1Nc1c2ccccc2nc2ccccc12)S(C)(=O)=O Show InChI InChI=1S/C21H18N2O3S/c1-26-20-13-14(27(2,24)25)11-12-19(20)23-21-15-7-3-5-9-17(15)22-18-10-6-4-8-16(18)21/h3-13H,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Poison activity at recombinant human topoisomerase 2beta using pBR322 plasmid as substrate after 30 mins by ethidium bromide staining based agarose g... |
J Med Chem 61: 8947-8980 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01202
BindingDB Entry DOI: 10.7270/Q2H998M5 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
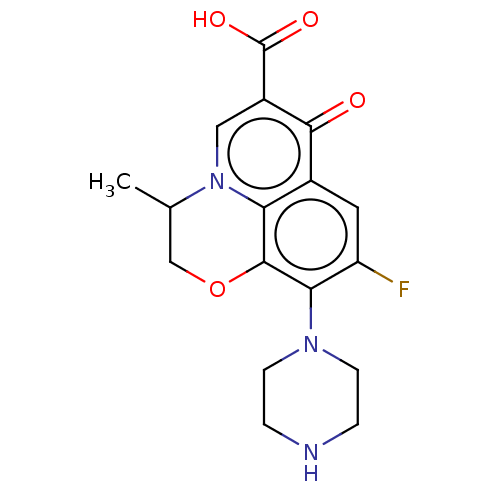
(Homo sapiens (Human)) | BDBM50229966
(DESMETHYL-OFLOXACIN | Desmethyl-Ofloxacin (5%))Show SMILES CC1COc2c(N3CCNCC3)c(F)cc3c2n1cc(C(O)=O)c3=O Show InChI InChI=1S/C17H18FN3O4/c1-9-8-25-16-13-10(15(22)11(17(23)24)7-21(9)13)6-12(18)14(16)20-4-2-19-3-5-20/h6-7,9,19H,2-5,8H2,1H3,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
The inhibitory activity was measured for the supercoiling activity of DNA gyrase isolated from Escherichia coli K-12 C600. |
J Med Chem 35: 94-9 (1992)
BindingDB Entry DOI: 10.7270/Q2D79DNN |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
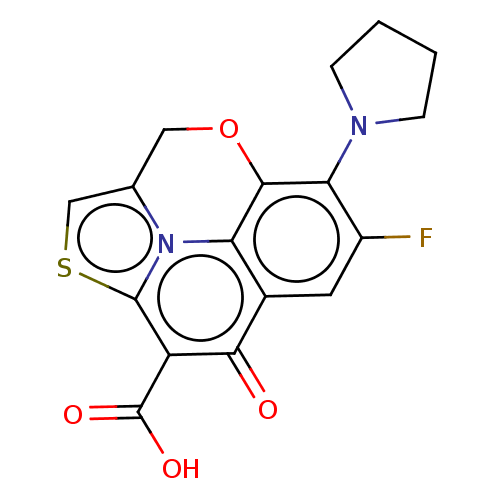
(Homo sapiens (Human)) | BDBM50229965
(CHEMBL174892)Show SMILES OC(=O)c1c2scc3COc4c(N5CCCC5)c(F)cc(c4n23)c1=O Show InChI InChI=1S/C17H13FN2O4S/c18-10-5-9-12-15(13(10)19-3-1-2-4-19)24-6-8-7-25-16(20(8)12)11(14(9)21)17(22)23/h5,7H,1-4,6H2,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
The inhibitory activity was measured for the supercoiling activity of DNA gyrase isolated from Escherichia coli K-12 C600. |
J Med Chem 35: 94-9 (1992)
BindingDB Entry DOI: 10.7270/Q2D79DNN |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
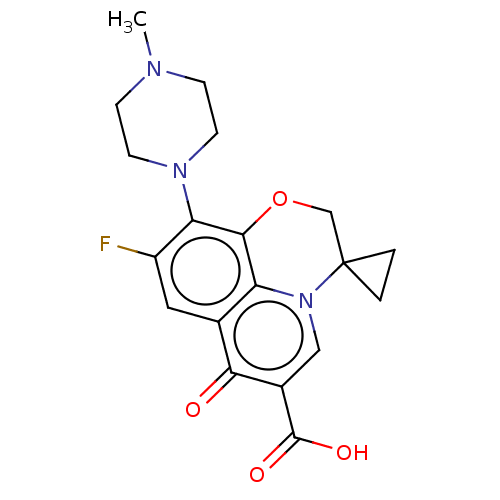
(Homo sapiens (Human)) | BDBM50227628
(CHEMBL47311)Show SMILES CN1CCN(CC1)c1c(F)cc2c3c1OCC1(CC1)n3cc(C(O)=O)c2=O Show InChI InChI=1S/C19H20FN3O4/c1-21-4-6-22(7-5-21)15-13(20)8-11-14-17(15)27-10-19(2-3-19)23(14)9-12(16(11)24)18(25)26/h8-9H,2-7,10H2,1H3,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling-Winthrop Research Institute
Curated by ChEMBL
| Assay Description
Gyrase inhibitory activity against Escherichia coli |
J Med Chem 31: 1694-7 (1988)
BindingDB Entry DOI: 10.7270/Q2RX9F84 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
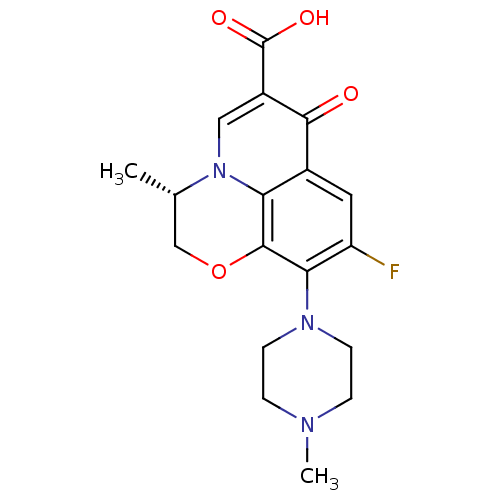
(Homo sapiens (Human)) | BDBM50366826
(DR-3355 | Floxacin | Iquix | LEVOFLOXACIN | Levaqu...)Show SMILES C[C@H]1COc2c(N3CCN(C)CC3)c(F)cc3c2n1cc(C(O)=O)c3=O |r| Show InChI InChI=1S/C18H20FN3O4/c1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21/h7-8,10H,3-6,9H2,1-2H3,(H,24,25)/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 2.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling-Winthrop Research Institute
Curated by ChEMBL
| Assay Description
Gyrase inhibitory activity against Escherichia coli |
J Med Chem 31: 1694-7 (1988)
BindingDB Entry DOI: 10.7270/Q2RX9F84 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50045004
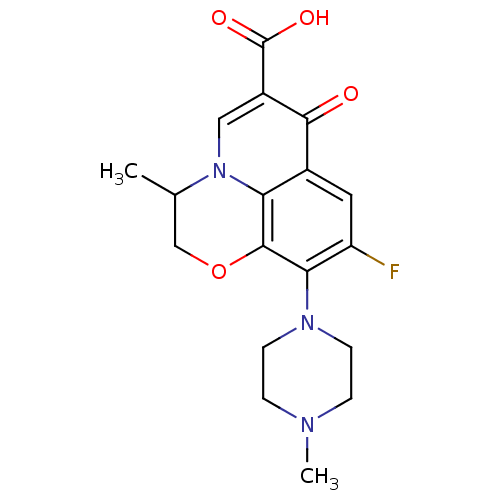
(9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-ox...)Show SMILES CC1COc2c(N3CCN(C)CC3)c(F)cc3c2n1cc(C(O)=O)c3=O Show InChI InChI=1S/C18H20FN3O4/c1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21/h7-8,10H,3-6,9H2,1-2H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling-Winthrop Research Institute
Curated by ChEMBL
| Assay Description
Gyrase inhibitory activity against Escherichia coli |
J Med Chem 31: 1694-7 (1988)
BindingDB Entry DOI: 10.7270/Q2RX9F84 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM87351
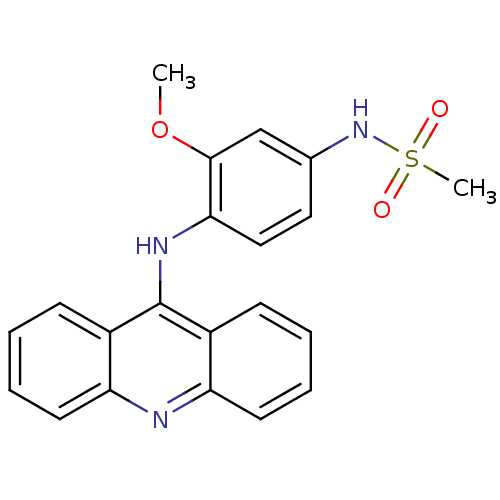
(Amsacrine hydrochloride | CHEMBL43 | MLS002153376 ...)Show SMILES COc1cc(NS(C)(=O)=O)ccc1Nc1c2ccccc2nc2ccccc12 Show InChI InChI=1S/C21H19N3O3S/c1-27-20-13-14(24-28(2,25)26)11-12-19(20)23-21-15-7-3-5-9-17(15)22-18-10-6-4-8-16(18)21/h3-13,24H,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of human topoisomerase-2B after 2 hrs by ELISA |
Eur J Med Chem 150: 403-418 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.026
BindingDB Entry DOI: 10.7270/Q2MS3WCZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50586362
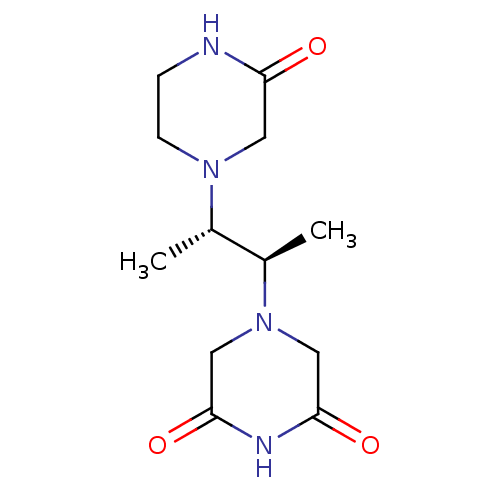
(CHEMBL5083245)Show SMILES C[C@@H]([C@@H](C)N1CC(=O)NC(=O)C1)N1CCNC(=O)C1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human TOP2B assessed as reduction in relaxation of supercoiled DNA using kDNA as substrate incubated for 30 min |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02157
BindingDB Entry DOI: 10.7270/Q2QJ7N6V |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM22984

((8S,10S)-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-me...)Show SMILES COc1cccc2C(=O)c3c(O)c4C[C@](O)(C[C@H](O[C@H]5C[C@H](N)[C@H](O)[C@H](C)O5)c4c(O)c3C(=O)c12)C(=O)CO |r| Show InChI InChI=1S/C27H29NO11/c1-10-22(31)13(28)6-17(38-10)39-15-8-27(36,16(30)9-29)7-12-19(15)26(35)21-20(24(12)33)23(32)11-4-3-5-14(37-2)18(11)25(21)34/h3-5,10,13,15,17,22,29,31,33,35-36H,6-9,28H2,1-2H3/t10-,13-,15-,17-,22+,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of human topoisomerase 2beta after 2 hrs at 37 degC by ELISA |
Eur J Med Chem 143: 1807-1825 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.075
BindingDB Entry DOI: 10.7270/Q2BC426J |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM22984

((8S,10S)-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-me...)Show SMILES COc1cccc2C(=O)c3c(O)c4C[C@](O)(C[C@H](O[C@H]5C[C@H](N)[C@H](O)[C@H](C)O5)c4c(O)c3C(=O)c12)C(=O)CO |r| Show InChI InChI=1S/C27H29NO11/c1-10-22(31)13(28)6-17(38-10)39-15-8-27(36,16(30)9-29)7-12-19(15)26(35)21-20(24(12)33)23(32)11-4-3-5-14(37-2)18(11)25(21)34/h3-5,10,13,15,17,22,29,31,33,35-36H,6-9,28H2,1-2H3/t10-,13-,15-,17-,22+,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of human topoisomerase-2B after 2 hrs by ELISA |
Eur J Med Chem 150: 403-418 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.026
BindingDB Entry DOI: 10.7270/Q2MS3WCZ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
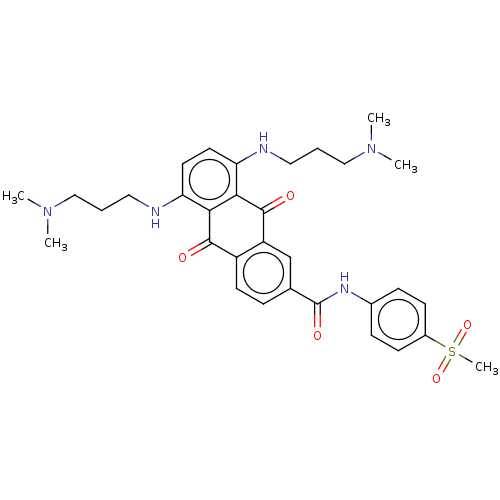
(Homo sapiens (Human)) | BDBM50520633
(CHEMBL4448353)Show SMILES CN(C)CCCNc1ccc(NCCCN(C)C)c2C(=O)c3cc(ccc3C(=O)c12)C(=O)Nc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C32H39N5O5S/c1-36(2)18-6-16-33-26-14-15-27(34-17-7-19-37(3)4)29-28(26)30(38)24-13-8-21(20-25(24)31(29)39)32(40)35-22-9-11-23(12-10-22)43(5,41)42/h8-15,20,33-34H,6-7,16-19H2,1-5H3,(H,35,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Poison activity at recombinant human topoisomerase 2beta using pBR322 plasmid as substrate after 30 mins by ethidium bromide staining based agarose g... |
J Med Chem 61: 8947-8980 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01202
BindingDB Entry DOI: 10.7270/Q2H998M5 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50520636
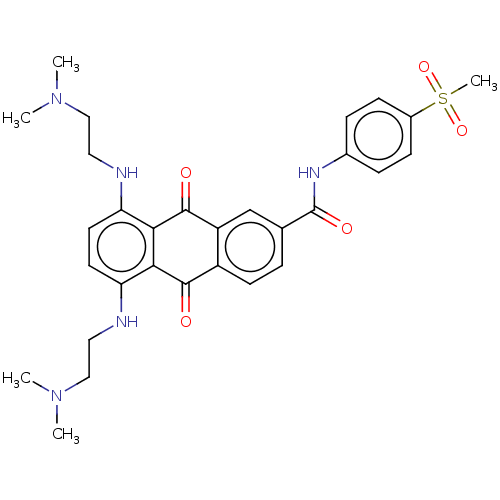
(CHEMBL4467805)Show SMILES CN(C)CCNc1ccc(NCCN(C)C)c2C(=O)c3cc(ccc3C(=O)c12)C(=O)Nc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C30H35N5O5S/c1-34(2)16-14-31-24-12-13-25(32-15-17-35(3)4)27-26(24)28(36)22-11-6-19(18-23(22)29(27)37)30(38)33-20-7-9-21(10-8-20)41(5,39)40/h6-13,18,31-32H,14-17H2,1-5H3,(H,33,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Poison activity at recombinant human topoisomerase 2beta using pBR322 plasmid as substrate after 30 mins by ethidium bromide staining based agarose g... |
J Med Chem 61: 8947-8980 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01202
BindingDB Entry DOI: 10.7270/Q2H998M5 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50474309
(CHEMBL331076)Show SMILES N[C@H]1CCCN(C1)c1c(F)cc2c(c1F)n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C18H19F2N3O3/c19-13-6-11-15(14(20)16(13)22-5-1-2-9(21)7-22)23(10-3-4-10)8-12(17(11)24)18(25)26/h6,8-10H,1-5,7,21H2,(H,25,26)/t9-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition effect on Escherichia coli Wild Type DNA gyrase Supercoiling activity |
J Med Chem 46: 3655-61 (2003)
Article DOI: 10.1021/jm030272n
BindingDB Entry DOI: 10.7270/Q2R2144Z |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50464488
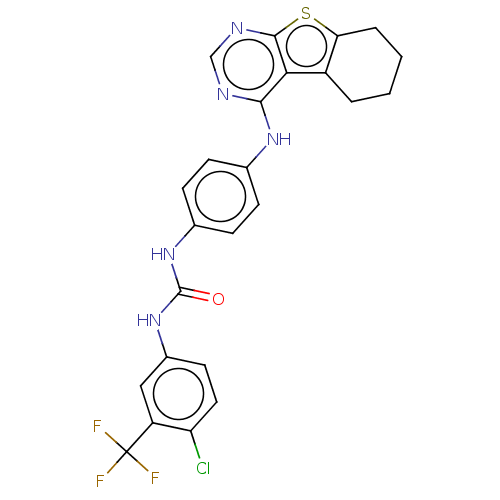
(CHEMBL4278922)Show SMILES FC(F)(F)c1cc(NC(=O)Nc2ccc(Nc3ncnc4sc5CCCCc5c34)cc2)ccc1Cl Show InChI InChI=1S/C24H19ClF3N5OS/c25-18-10-9-15(11-17(18)24(26,27)28)33-23(34)32-14-7-5-13(6-8-14)31-21-20-16-3-1-2-4-19(16)35-22(20)30-12-29-21/h5-12H,1-4H2,(H,29,30,31)(H2,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of human topoisomerase 2beta after 2 hrs at 37 degC by ELISA |
Eur J Med Chem 143: 1807-1825 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.075
BindingDB Entry DOI: 10.7270/Q2BC426J |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50135927
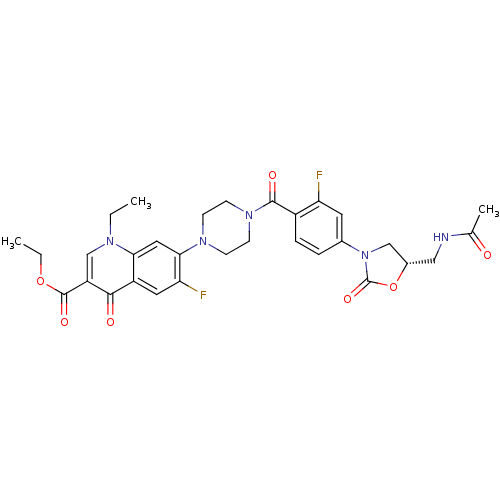
(7-(4-{4-[(S)-5-(Acetylamino-methyl)-2-oxo-oxazolid...)Show SMILES CCOC(=O)c1cn(CC)c2cc(N3CCN(CC3)C(=O)c3ccc(cc3F)N3C[C@H](CNC(C)=O)OC3=O)c(F)cc2c1=O Show InChI InChI=1S/C31H33F2N5O7/c1-4-35-17-23(30(42)44-5-2)28(40)22-13-25(33)27(14-26(22)35)36-8-10-37(11-9-36)29(41)21-7-6-19(12-24(21)32)38-16-20(45-31(38)43)15-34-18(3)39/h6-7,12-14,17,20H,4-5,8-11,15-16H2,1-3H3,(H,34,39)/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vicuron Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Escherichia coli by Escherichia coli DNA gyrase gel-based supercoil assay |
Bioorg Med Chem Lett 13: 4213-6 (2003)
BindingDB Entry DOI: 10.7270/Q2MP52PS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50586360
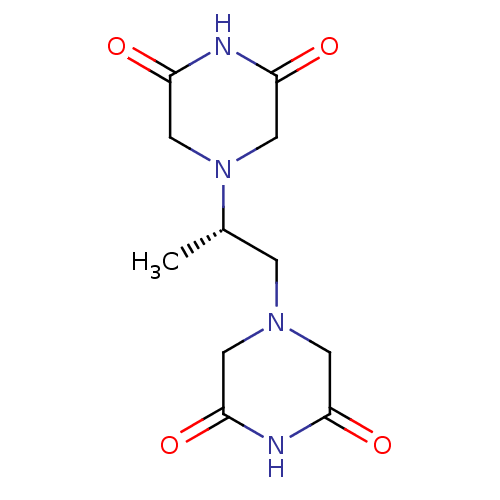
(ADR-529 | CHEBI:50223 | Cardioxane | DEXRAZOXANE |...) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human TOP2B assessed as reduction in relaxation of supercoiled DNA using kDNA as substrate incubated for 30 min |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02157
BindingDB Entry DOI: 10.7270/Q2QJ7N6V |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50473500
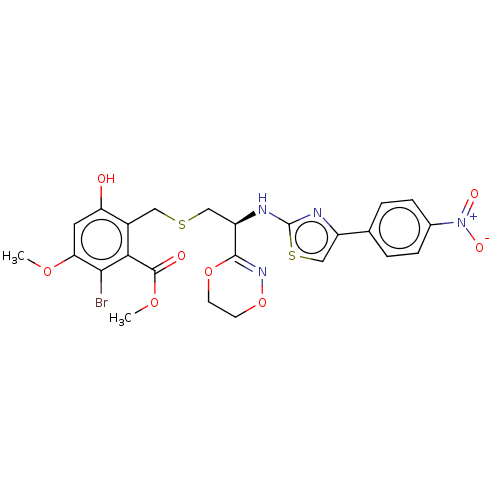
(CHEMBL158264)Show SMILES COC(=O)c1c(Br)c(OC)cc(O)c1CSC[C@@H](Nc1nc(cs1)-c1ccc(cc1)[N+]([O-])=O)C1=NOCCO1 |t:36| Show InChI InChI=1S/C24H23BrN4O8S2/c1-34-19-9-18(30)15(20(21(19)25)23(31)35-2)10-38-11-17(22-28-37-8-7-36-22)27-24-26-16(12-39-24)13-3-5-14(6-4-13)29(32)33/h3-6,9,12,17,30H,7-8,10-11H2,1-2H3,(H,26,27)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antibacterial activity against Micrococcus luteus DNA gyrase. |
J Med Chem 44: 619-26 (2001)
Article DOI: 10.1021/jm0010623
BindingDB Entry DOI: 10.7270/Q2T43WT3 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
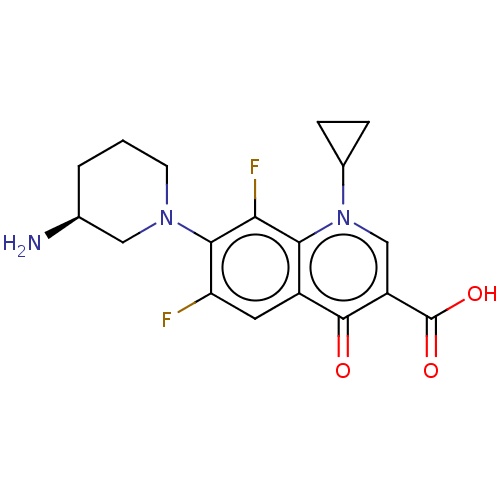
(Homo sapiens (Human)) | BDBM50474309

(CHEMBL331076)Show SMILES N[C@H]1CCCN(C1)c1c(F)cc2c(c1F)n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C18H19F2N3O3/c19-13-6-11-15(14(20)16(13)22-5-1-2-9(21)7-22)23(10-3-4-10)8-12(17(11)24)18(25)26/h6,8-10H,1-5,7,21H2,(H,25,26)/t9-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli Quinolone resistant gyrase Supercoiling activity |
J Med Chem 46: 3655-61 (2003)
Article DOI: 10.1021/jm030272n
BindingDB Entry DOI: 10.7270/Q2R2144Z |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50366305
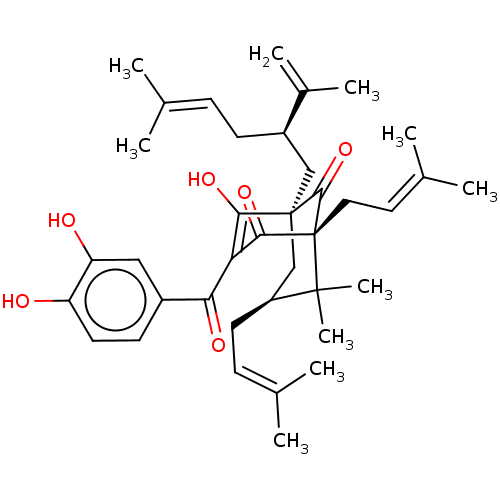
(CHEBI:70328 | CHEMBL445599)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6@H](-[#6][C@]12[#6]-[#6@H](-[#6]\[#6]=[#6](\[#6])-[#6])C([#6])([#6])[C@]([#6]\[#6]=[#6](\[#6])-[#6])([#6](=O)-[#6](-[#6](=O)-c3ccc(-[#8])c(-[#8])c3)=[#6]1-[#8])[#6]2=O)-[#6](-[#6])=[#6] |r,c:37,TLB:40:39:26.37.24:15.8.9| Show InChI InChI=1S/C38H50O6/c1-22(2)11-13-27(25(7)8)20-37-21-28(15-12-23(3)4)36(9,10)38(35(37)44,18-17-24(5)6)34(43)31(33(37)42)32(41)26-14-16-29(39)30(40)19-26/h11-12,14,16-17,19,27-28,39-40,42H,7,13,15,18,20-21H2,1-6,8-10H3/t27-,28+,37+,38-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
European Biomedical Research Institute of Salerno (EBRIS)
Curated by ChEMBL
| Assay Description
Inhibition of human topoisomerase 2beta-mediated relaxation of supercoiled pSG483 DNA after 30 mins by ethidium bromide staining based agarose gel el... |
J Nat Prod 82: 2768-2779 (2019)
Article DOI: 10.1021/acs.jnatprod.9b00382
BindingDB Entry DOI: 10.7270/Q2QN6B3F |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50471425
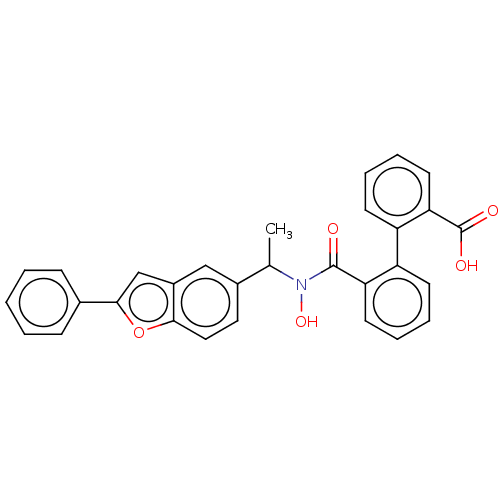
(CHEMBL110041)Show SMILES CC(N(O)C(=O)c1ccccc1-c1ccccc1C(O)=O)c1ccc2oc(cc2c1)-c1ccccc1 Show InChI InChI=1S/C30H23NO5/c1-19(21-15-16-27-22(17-21)18-28(36-27)20-9-3-2-4-10-20)31(35)29(32)25-13-7-5-11-23(25)24-12-6-8-14-26(24)30(33)34/h2-19,35H,1H3,(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.21E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of supercoiling activity of DNA gyrase in Escherichia coli |
J Med Chem 40: 3292-6 (1997)
Article DOI: 10.1021/jm9701583
BindingDB Entry DOI: 10.7270/Q2FJ2KHP |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50554356
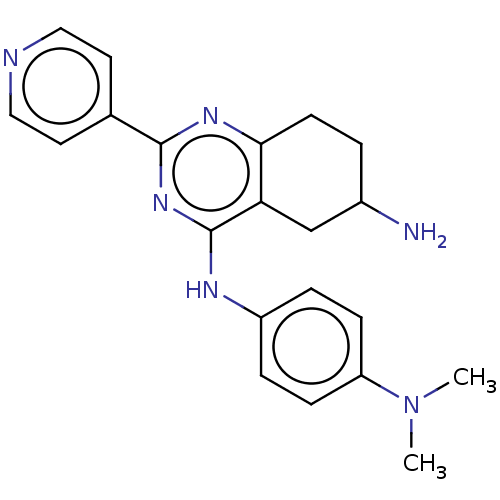
(CHEMBL4758733)Show SMILES CN(C)c1ccc(Nc2nc(nc3CCC(N)Cc23)-c2ccncc2)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant wild-type human topoisomerase 2beta expressed in Saccharomyces cerevisiae using supercoiled pBR322 DNA as substrate measure... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00774
BindingDB Entry DOI: 10.7270/Q26H4N2X |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50471426
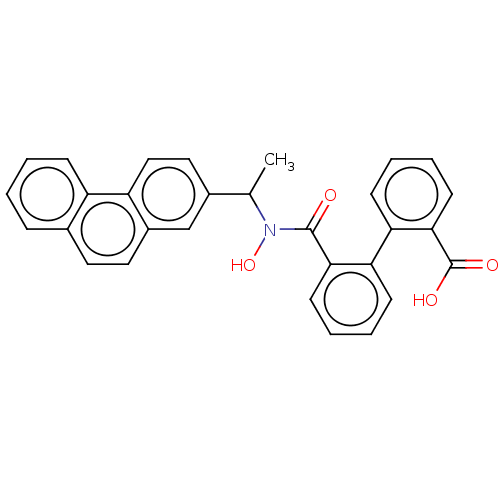
(CHEMBL110367)Show SMILES CC(N(O)C(=O)c1ccccc1-c1ccccc1C(O)=O)c1ccc2c(ccc3ccccc23)c1 Show InChI InChI=1S/C30H23NO4/c1-19(21-16-17-24-22(18-21)15-14-20-8-2-3-9-23(20)24)31(35)29(32)27-12-6-4-10-25(27)26-11-5-7-13-28(26)30(33)34/h2-19,35H,1H3,(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of supercoiling activity of DNA gyrase in Escherichia coli |
J Med Chem 40: 3292-6 (1997)
Article DOI: 10.1021/jm9701583
BindingDB Entry DOI: 10.7270/Q2FJ2KHP |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM21691
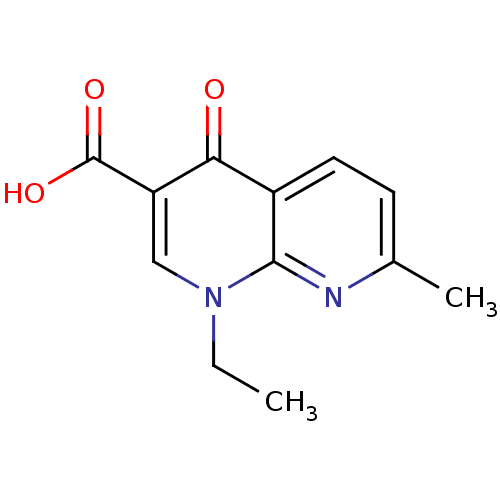
(1-ethyl-7-methyl-4-oxo-1,4-dihydro-1,8-naphthyridi...)Show InChI InChI=1S/C12H12N2O3/c1-3-14-6-9(12(16)17)10(15)8-5-4-7(2)13-11(8)14/h4-6H,3H2,1-2H3,(H,16,17) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.23E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of supercoiling activity of DNA gyrase in Escherichia coli |
J Med Chem 40: 3292-6 (1997)
Article DOI: 10.1021/jm9701583
BindingDB Entry DOI: 10.7270/Q2FJ2KHP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data