

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasopressin V2 receptor (Sus scrofa) | BDBM35667 (AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the inhibition to V2 subtype receptor using [3H]- (VS2) as radioligand at 3 nM and arginine-vasopressin at 2 microM in LLCPKI cells | Bioorg Med Chem Lett 7: 719-724 (1997) Article DOI: 10.1016/S0960-894X(97)00050-4 BindingDB Entry DOI: 10.7270/Q2QC03HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50291329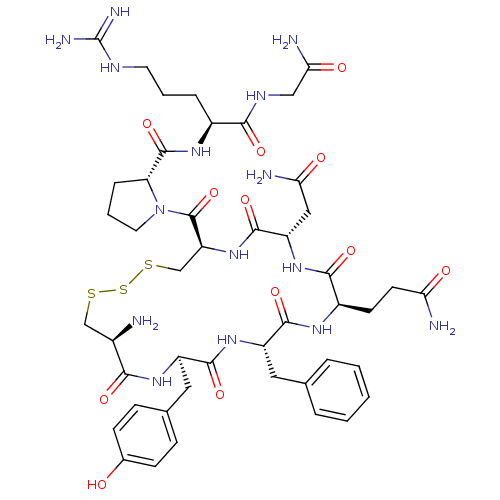 (8-arginine vasopressin trisulphide | CHEMBL267405) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the inhibition to V2 subtype receptor using [3H]- (VS2) as radioligand at 3 nM and arginine-vasopressin at 2 microM in LLCPKI cells | Bioorg Med Chem Lett 7: 719-724 (1997) Article DOI: 10.1016/S0960-894X(97)00050-4 BindingDB Entry DOI: 10.7270/Q2QC03HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50122716 (5-(Piperidin-4-ylmethoxy)-9-propyl-1,2,3,4-tetrahy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Caen Curated by ChEMBL | Assay Description Ability to displace [3H]AVP from Vasopressin V2 receptor | J Med Chem 46: 138-47 (2002) Article DOI: 10.1021/jm020954v BindingDB Entry DOI: 10.7270/Q2J67G8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50281512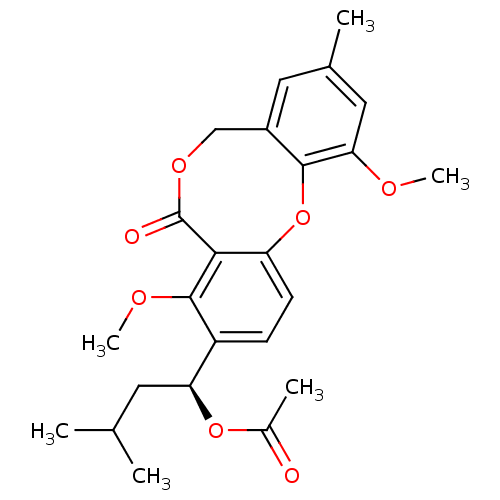 (Acetic acid (S)-1-(4,11-dimethoxy-9-methyl-5-oxo-5...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit binding of arginine vasopressin to Vasopressin receptor (V1 subtype) | Bioorg Med Chem Lett 3: 337-340 (1993) Article DOI: 10.1016/S0960-894X(01)80905-7 BindingDB Entry DOI: 10.7270/Q28052J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50281511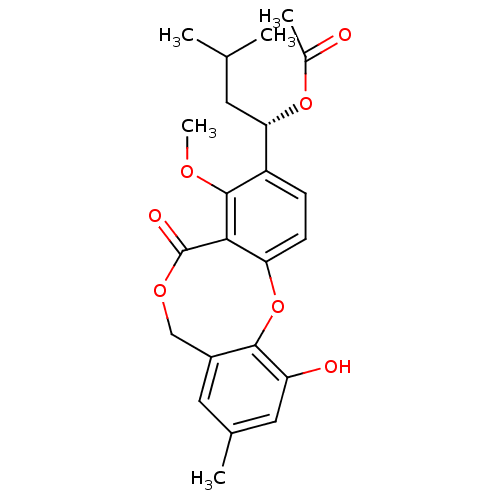 (Acetic acid (S)-1-(11-hydroxy-4-methoxy-9-methyl-5...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit binding of arginine vasopressin to Vasopressin receptor (V1 subtype) | Bioorg Med Chem Lett 3: 337-340 (1993) Article DOI: 10.1016/S0960-894X(01)80905-7 BindingDB Entry DOI: 10.7270/Q28052J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||