

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50474105 (CHEMBL43341) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Marburg Curated by ChEMBL | Assay Description pKi value expresses binding affinity against thermolysin pKi = -logKi (where Ki is in mol/L) | J Med Chem 45: 1585-97 (2002) Article DOI: 10.1021/jm011039x BindingDB Entry DOI: 10.7270/Q25B057T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50006111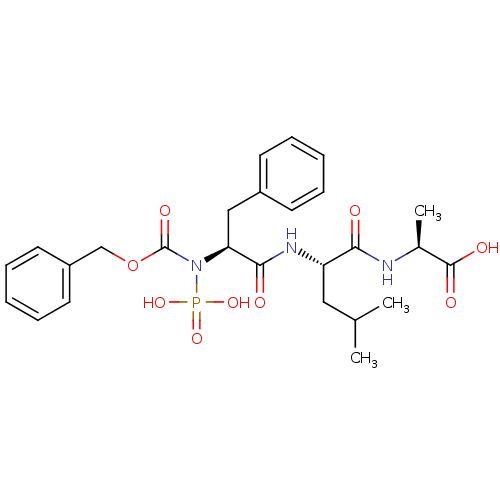 ((ZFPLA) 2-[2-(2-Benzyloxycarbonylamino-3-phenyl-pr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibitory constant against thermolysin. | J Med Chem 35: 1671-84 (1992) BindingDB Entry DOI: 10.7270/Q2DR2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50474138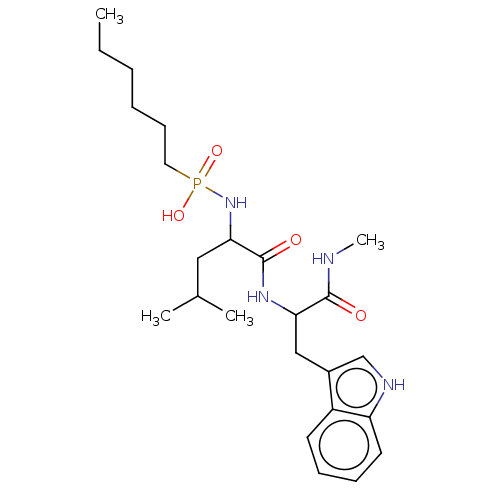 (CHEMBL38873) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Marburg Curated by ChEMBL | Assay Description pKi value expresses binding affinity against thermolysin pKi = -logKi (where Ki is in mol/L) | J Med Chem 45: 1585-97 (2002) Article DOI: 10.1021/jm011039x BindingDB Entry DOI: 10.7270/Q25B057T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50070927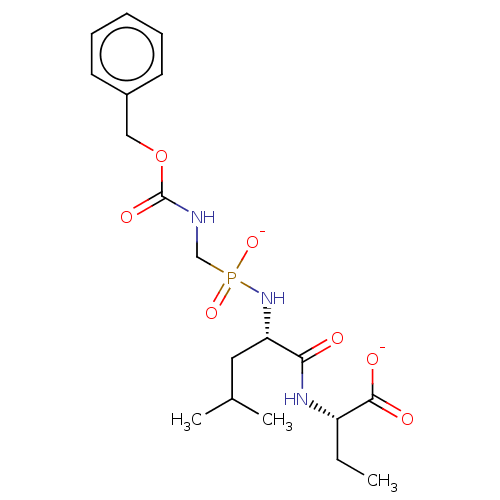 (CHEMBL3409520) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus TLN using 2-furanacryloyl-Gly-Leu-NH2 substrate by biochemical assay | Eur J Med Chem 90: 897-915 (2015) Article DOI: 10.1016/j.ejmech.2014.11.056 BindingDB Entry DOI: 10.7270/Q2FJ2JFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50070925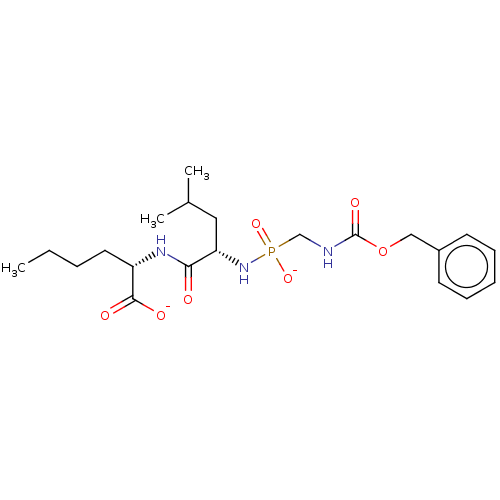 (CHEMBL3409522) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus TLN using 2-furanacryloyl-Gly-Leu-NH2 substrate by biochemical assay | Eur J Med Chem 90: 897-915 (2015) Article DOI: 10.1016/j.ejmech.2014.11.056 BindingDB Entry DOI: 10.7270/Q2FJ2JFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50070926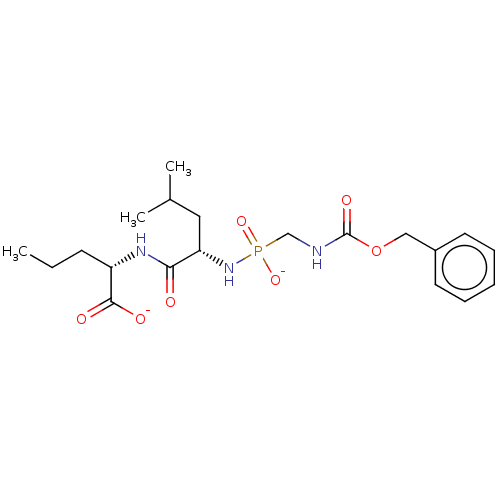 (CHEMBL3409521) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus TLN using 2-furanacryloyl-Gly-Leu-NH2 substrate by biochemical assay | Eur J Med Chem 90: 897-915 (2015) Article DOI: 10.1016/j.ejmech.2014.11.056 BindingDB Entry DOI: 10.7270/Q2FJ2JFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50070924 (CHEMBL3409523) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus TLN using 2-furanacryloyl-Gly-Leu-NH2 substrate by biochemical assay | Eur J Med Chem 90: 897-915 (2015) Article DOI: 10.1016/j.ejmech.2014.11.056 BindingDB Entry DOI: 10.7270/Q2FJ2JFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50006110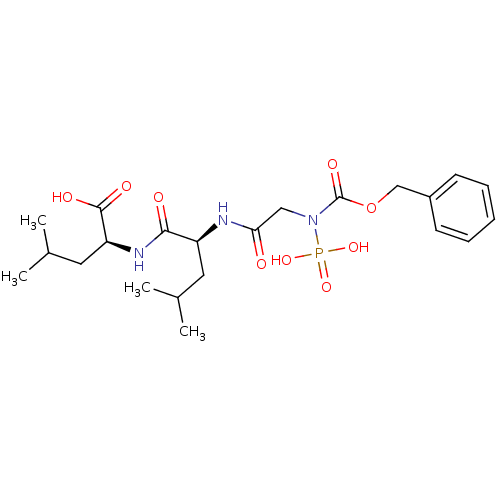 ((ZGPLA) 2-[2-(2-Benzyloxycarbonylamino-acetylamino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibitory constant against thermolysin. | J Med Chem 35: 1671-84 (1992) BindingDB Entry DOI: 10.7270/Q2DR2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50474141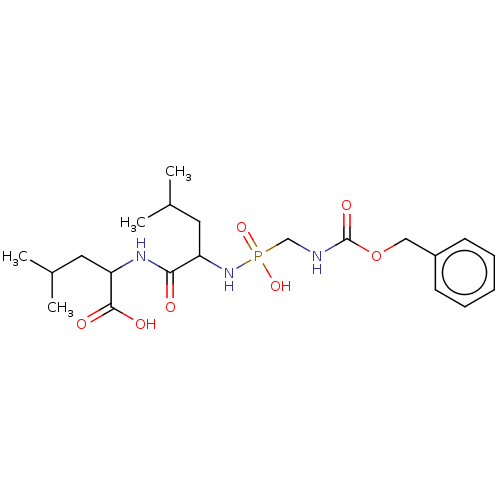 (CHEMBL43176) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 9.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Marburg Curated by ChEMBL | Assay Description pKi value expresses binding affinity against thermolysin pKi = -logKi (where Ki is in mol/L) | J Med Chem 45: 1585-97 (2002) Article DOI: 10.1021/jm011039x BindingDB Entry DOI: 10.7270/Q25B057T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50070937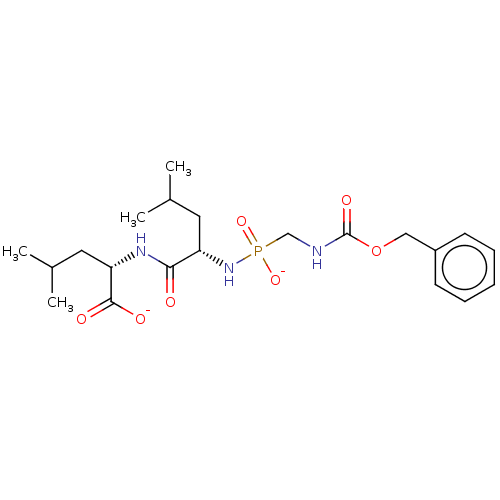 (CHEMBL3409510) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus TLN using 2-furanacryloyl-Gly-Leu-NH2 substrate by biochemical assay | Eur J Med Chem 90: 897-915 (2015) Article DOI: 10.1016/j.ejmech.2014.11.056 BindingDB Entry DOI: 10.7270/Q2FJ2JFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50121452 ((S)-2-{(S)-2-[(Benzyloxycarbonylamino-methyl)-hydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Competitive inhibition of thermolysin evaluated using the substrate FAGLA (Sigma); Competitive binding observed | Bioorg Med Chem Lett 12: 3625-7 (2002) BindingDB Entry DOI: 10.7270/Q269744P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50474083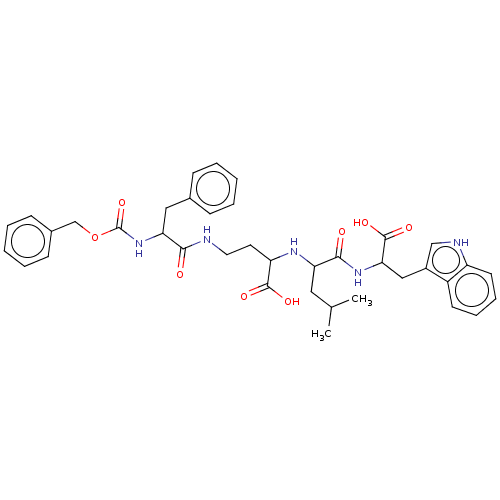 (CHEMBL41348) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Marburg Curated by ChEMBL | Assay Description pKi value expresses binding affinity against thermolysin pKi = -logKi (where Ki is in mol/L) | J Med Chem 45: 1585-97 (2002) Article DOI: 10.1021/jm011039x BindingDB Entry DOI: 10.7270/Q25B057T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50321113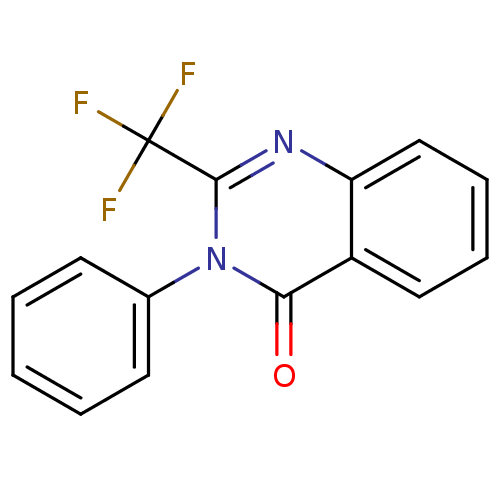 (3-Phenyl-2-(trifluoromethyl)quinazolin-4(3H)-one |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Troms£ Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus thermolysin after 15 mins by microplate fluorescence analysis in presence of 0.5 to 2 mM substrate FaGLa | Bioorg Med Chem 18: 4317-27 (2010) Article DOI: 10.1016/j.bmc.2010.04.083 BindingDB Entry DOI: 10.7270/Q2QV3MP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50070922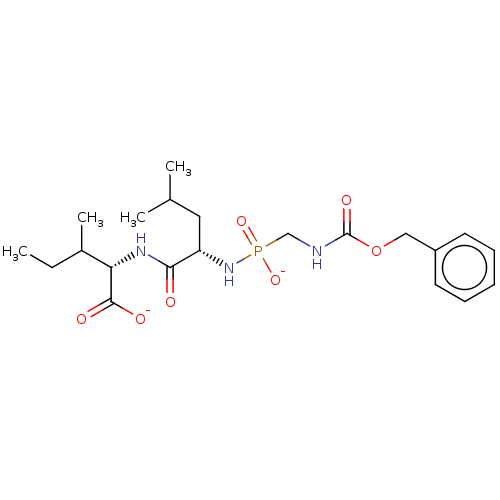 (CHEMBL3409525) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus TLN using 2-furanacryloyl-Gly-Leu-NH2 substrate by biochemical assay | Eur J Med Chem 90: 897-915 (2015) Article DOI: 10.1016/j.ejmech.2014.11.056 BindingDB Entry DOI: 10.7270/Q2FJ2JFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50474089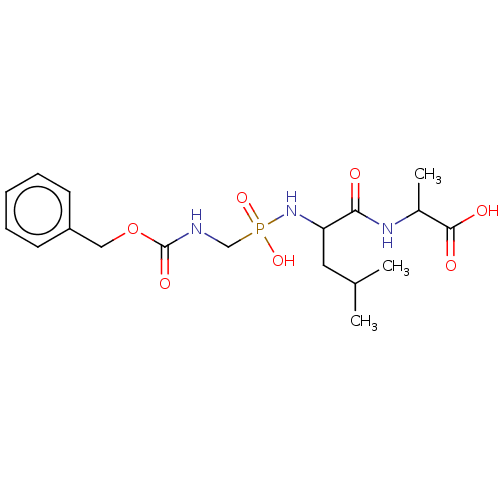 (CHEMBL43228) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 16.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Marburg Curated by ChEMBL | Assay Description pKi value expresses binding affinity against thermolysin pKi = -logKi (where Ki is in mol/L) | J Med Chem 45: 1585-97 (2002) Article DOI: 10.1021/jm011039x BindingDB Entry DOI: 10.7270/Q25B057T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50474119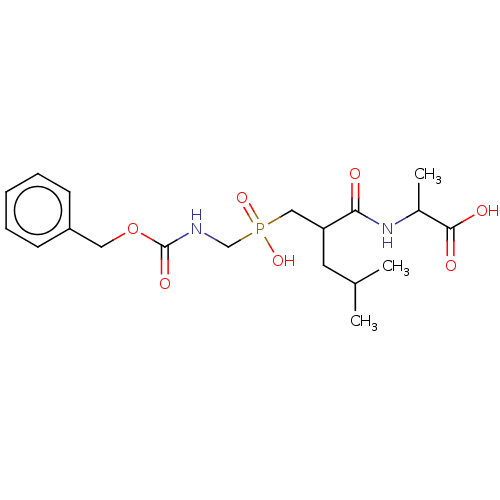 (CHEMBL40208) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 18.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Marburg Curated by ChEMBL | Assay Description pKi value expresses binding affinity against thermolysin pKi = -logKi (where Ki is in mol/L) | J Med Chem 45: 1585-97 (2002) Article DOI: 10.1021/jm011039x BindingDB Entry DOI: 10.7270/Q25B057T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50396788 (CHEMBL2172738) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus TLN using 2-furanacryloyl-Gly-Leu-NH2 substrate by biochemical assay | Eur J Med Chem 90: 897-915 (2015) Article DOI: 10.1016/j.ejmech.2014.11.056 BindingDB Entry DOI: 10.7270/Q2FJ2JFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50474137 (CHEMBL41500) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 19.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Marburg Curated by ChEMBL | Assay Description pKi value expresses binding affinity against thermolysin pKi = -logKi (where Ki is in mol/L) | J Med Chem 45: 1585-97 (2002) Article DOI: 10.1021/jm011039x BindingDB Entry DOI: 10.7270/Q25B057T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50396788 (CHEMBL2172738) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The State University of New York Curated by ChEMBL | Assay Description Binding affinity to Bacillus thermoproteolyticus thermolysin by UV-VIS spectrophotometric analysis in presence of 2-furanacryloyl-Gly-Leu-NH2 | J Med Chem 55: 8283-302 (2012) Article DOI: 10.1021/jm300472k BindingDB Entry DOI: 10.7270/Q27M092M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50070917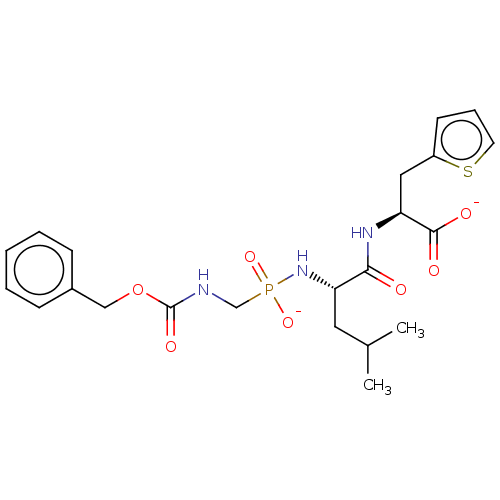 (CHEMBL3409528) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus TLN using 2-furanacryloyl-Gly-Leu-NH2 substrate by biochemical assay | Eur J Med Chem 90: 897-915 (2015) Article DOI: 10.1016/j.ejmech.2014.11.056 BindingDB Entry DOI: 10.7270/Q2FJ2JFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50070931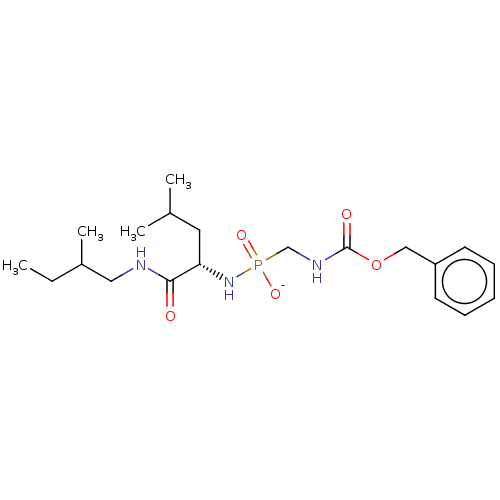 (CHEMBL3409516) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus TLN using 2-furanacryloyl-Gly-Leu-NH2 substrate by biochemical assay | Eur J Med Chem 90: 897-915 (2015) Article DOI: 10.1016/j.ejmech.2014.11.056 BindingDB Entry DOI: 10.7270/Q2FJ2JFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50251742 ((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibitory constant against thermolysin. | J Med Chem 35: 1671-84 (1992) BindingDB Entry DOI: 10.7270/Q2DR2W3S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50474108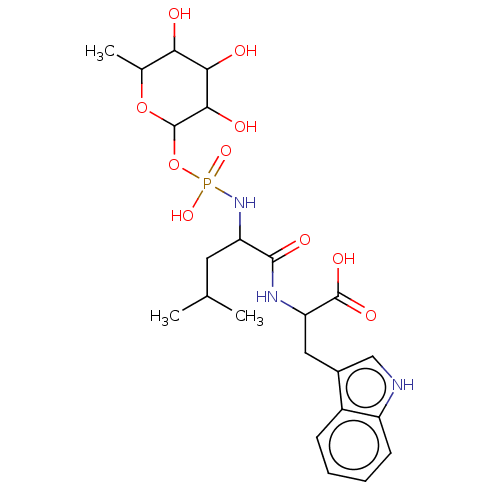 (CHEMBL41289) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 28.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Marburg Curated by ChEMBL | Assay Description pKi value expresses binding affinity against thermolysin pKi = -logKi (where Ki is in mol/L) | J Med Chem 45: 1585-97 (2002) Article DOI: 10.1021/jm011039x BindingDB Entry DOI: 10.7270/Q25B057T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50070938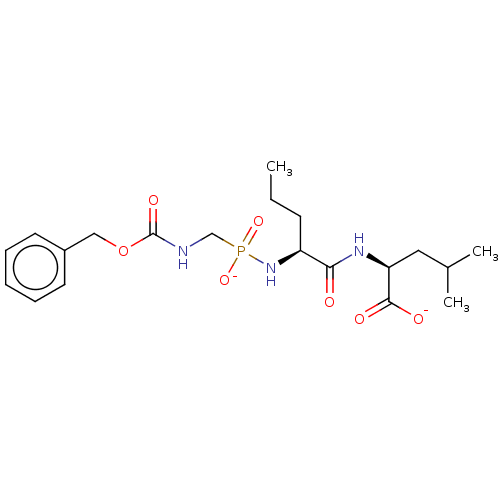 (CHEMBL3409509) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus TLN using 2-furanacryloyl-Gly-Leu-NH2 substrate by biochemical assay | Eur J Med Chem 90: 897-915 (2015) Article DOI: 10.1016/j.ejmech.2014.11.056 BindingDB Entry DOI: 10.7270/Q2FJ2JFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50442257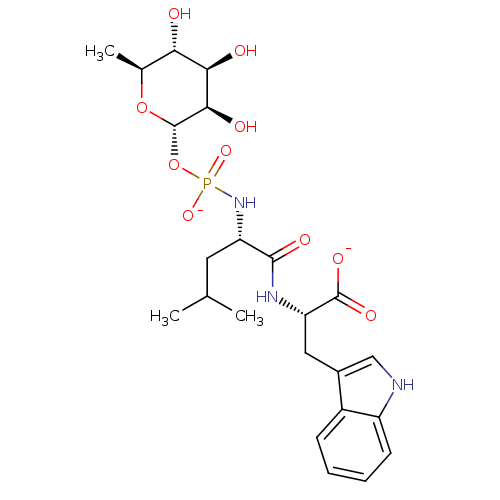 (CHEMBL2028192) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science and Technology Normal University Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus thermolysin using FA-Fly-Leu-NH2 as substrate at pH 7.5 after 15 mins by Henderson plot analysis | Bioorg Med Chem 21: 6778-87 (2013) Article DOI: 10.1016/j.bmc.2013.07.052 BindingDB Entry DOI: 10.7270/Q2CF9RJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50070923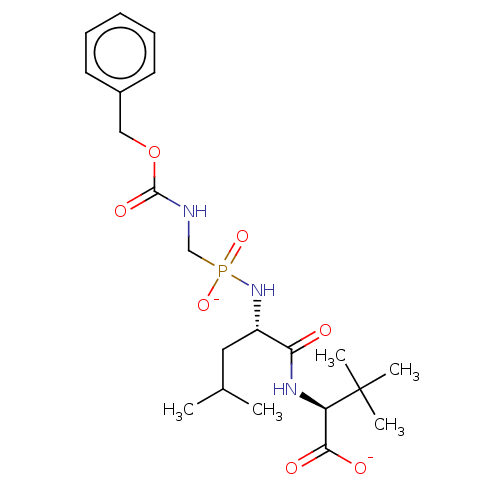 (CHEMBL3409524) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus TLN using 2-furanacryloyl-Gly-Leu-NH2 substrate by biochemical assay | Eur J Med Chem 90: 897-915 (2015) Article DOI: 10.1016/j.ejmech.2014.11.056 BindingDB Entry DOI: 10.7270/Q2FJ2JFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50442257 (CHEMBL2028192) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science and Technology Normal University Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus thermolysin | Bioorg Med Chem 21: 6778-87 (2013) Article DOI: 10.1016/j.bmc.2013.07.052 BindingDB Entry DOI: 10.7270/Q2CF9RJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50422014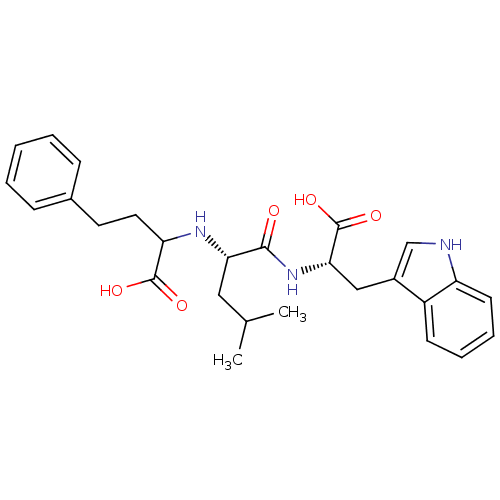 (CHEMBL319299) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Marburg Curated by ChEMBL | Assay Description pKi value expresses binding affinity against thermolysin pKi = -logKi (where Ki is in mol/L) | J Med Chem 45: 1585-97 (2002) Article DOI: 10.1021/jm011039x BindingDB Entry DOI: 10.7270/Q25B057T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50070933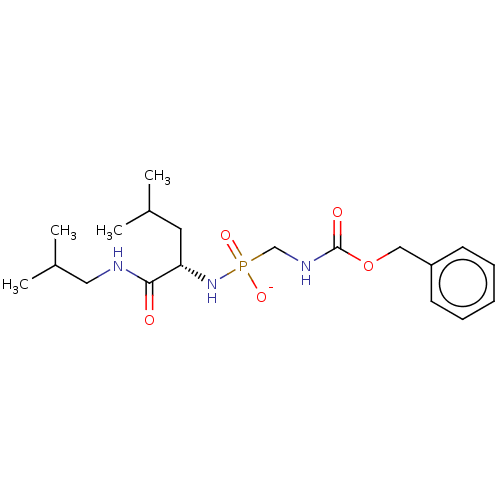 (CHEMBL3409514) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus TLN using 2-furanacryloyl-Gly-Leu-NH2 substrate by biochemical assay | Eur J Med Chem 90: 897-915 (2015) Article DOI: 10.1016/j.ejmech.2014.11.056 BindingDB Entry DOI: 10.7270/Q2FJ2JFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50070920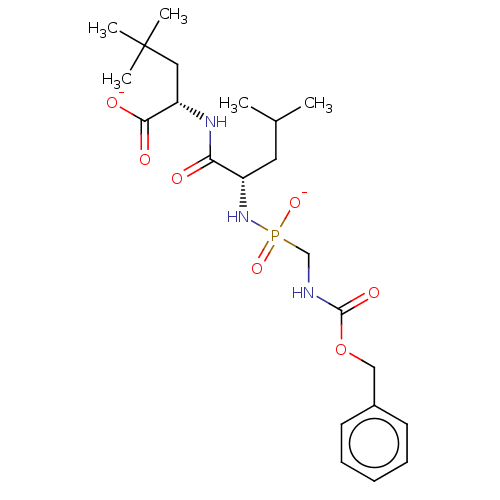 (CHEMBL3409526) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus TLN using 2-furanacryloyl-Gly-Leu-NH2 substrate by biochemical assay | Eur J Med Chem 90: 897-915 (2015) Article DOI: 10.1016/j.ejmech.2014.11.056 BindingDB Entry DOI: 10.7270/Q2FJ2JFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50121454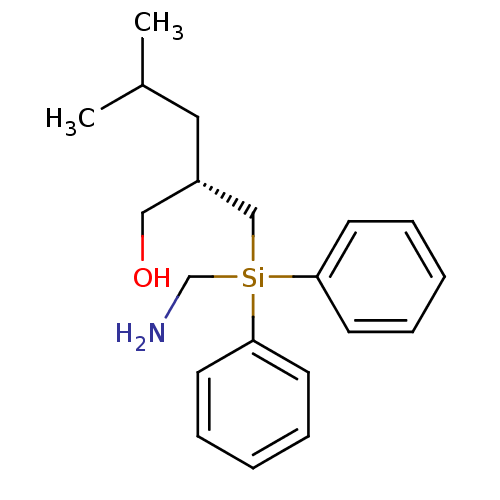 ((S)-2-[(Aminomethyl-diphenyl-silanyl)-methyl]-4-me...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Competitive inhibition of thermolysin evaluated using the substrate FAGLA (Sigma); Competitive binding observed | Bioorg Med Chem Lett 12: 3625-7 (2002) BindingDB Entry DOI: 10.7270/Q269744P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50121453 (CHEMBL334233 | Sodium; (S)-2-[(S)-2-({dihydroxy-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Competitive inhibition of thermolysin evaluated using the substrate FAGLA (Sigma); Competitive binding observed | Bioorg Med Chem Lett 12: 3625-7 (2002) BindingDB Entry DOI: 10.7270/Q269744P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50070930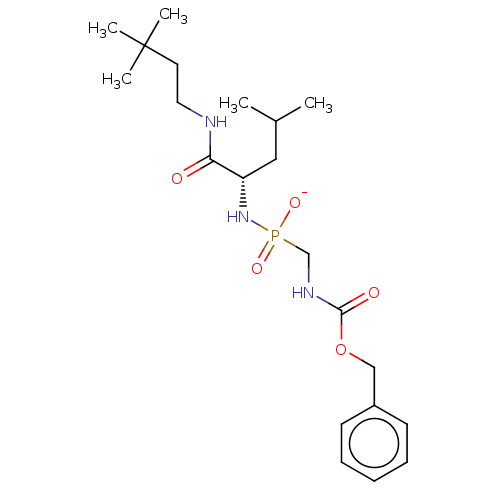 (CHEMBL3409517) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus TLN using 2-furanacryloyl-Gly-Leu-NH2 substrate by biochemical assay | Eur J Med Chem 90: 897-915 (2015) Article DOI: 10.1016/j.ejmech.2014.11.056 BindingDB Entry DOI: 10.7270/Q2FJ2JFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50474144 (CHEMBL287732) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 44.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Marburg Curated by ChEMBL | Assay Description pKi value expresses binding affinity against thermolysin pKi = -logKi (where Ki is in mol/L) | J Med Chem 45: 1585-97 (2002) Article DOI: 10.1021/jm011039x BindingDB Entry DOI: 10.7270/Q25B057T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50422014 (CHEMBL319299) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Tested for binding affinity against Thermolysin | J Med Chem 37: 1865-73 (1994) BindingDB Entry DOI: 10.7270/Q2474BG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50006117 ((CLT) 2-{1-[1-Carboxy-2-(1H-indol-3-yl)-ethylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibitory constant against thermolysin. | J Med Chem 35: 1671-84 (1992) BindingDB Entry DOI: 10.7270/Q2DR2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50474113 (CHEMBL289703) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 52.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Marburg Curated by ChEMBL | Assay Description pKi value expresses binding affinity against thermolysin pKi = -logKi (where Ki is in mol/L) | J Med Chem 45: 1585-97 (2002) Article DOI: 10.1021/jm011039x BindingDB Entry DOI: 10.7270/Q25B057T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50070918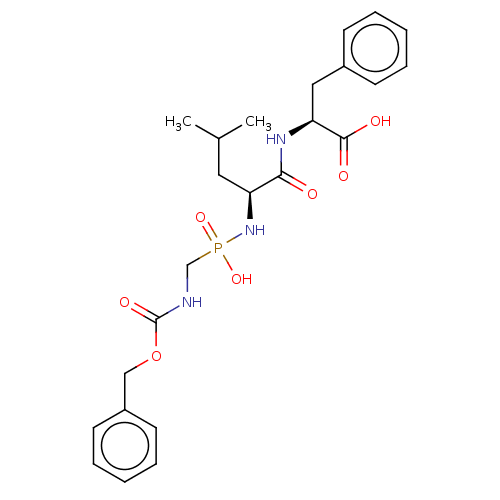 (CHEMBL3559407) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus TLN using 2-furanacryloyl-Gly-Leu-NH2 substrate by biochemical assay | Eur J Med Chem 90: 897-915 (2015) Article DOI: 10.1016/j.ejmech.2014.11.056 BindingDB Entry DOI: 10.7270/Q2FJ2JFC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50070934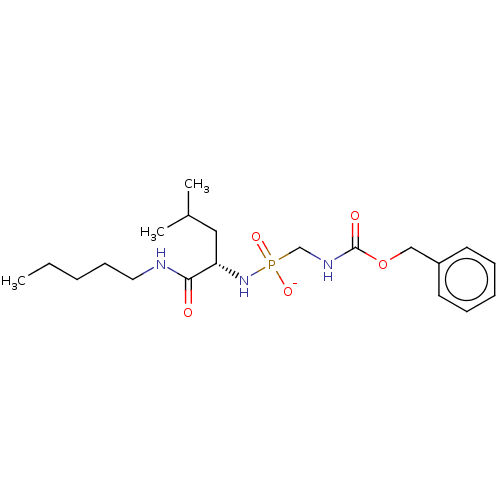 (CHEMBL3409513) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus TLN using 2-furanacryloyl-Gly-Leu-NH2 substrate by biochemical assay | Eur J Med Chem 90: 897-915 (2015) Article DOI: 10.1016/j.ejmech.2014.11.056 BindingDB Entry DOI: 10.7270/Q2FJ2JFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50070936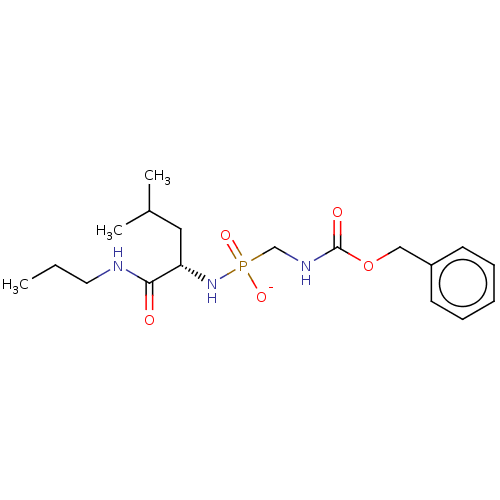 (CHEMBL3409511) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus TLN using 2-furanacryloyl-Gly-Leu-NH2 substrate by biochemical assay | Eur J Med Chem 90: 897-915 (2015) Article DOI: 10.1016/j.ejmech.2014.11.056 BindingDB Entry DOI: 10.7270/Q2FJ2JFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50474121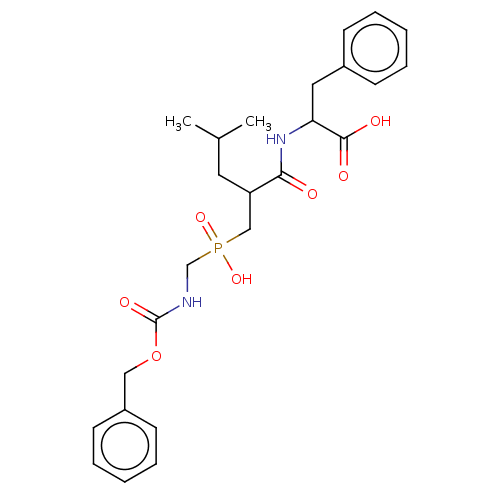 (CHEMBL436544) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 66.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Marburg Curated by ChEMBL | Assay Description pKi value expresses binding affinity against thermolysin pKi = -logKi (where Ki is in mol/L) | J Med Chem 45: 1585-97 (2002) Article DOI: 10.1021/jm011039x BindingDB Entry DOI: 10.7270/Q25B057T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50070935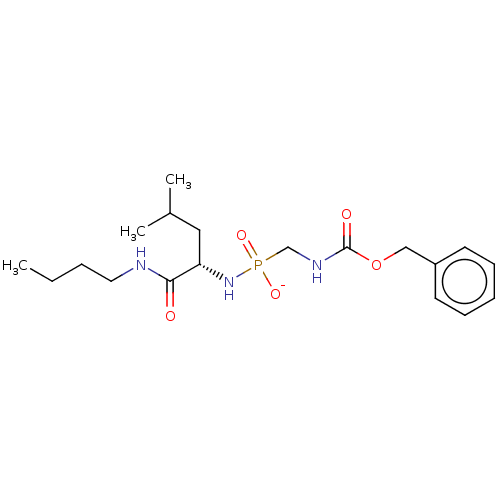 (CHEMBL3409512) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus TLN using 2-furanacryloyl-Gly-Leu-NH2 substrate by biochemical assay | Eur J Med Chem 90: 897-915 (2015) Article DOI: 10.1016/j.ejmech.2014.11.056 BindingDB Entry DOI: 10.7270/Q2FJ2JFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50070941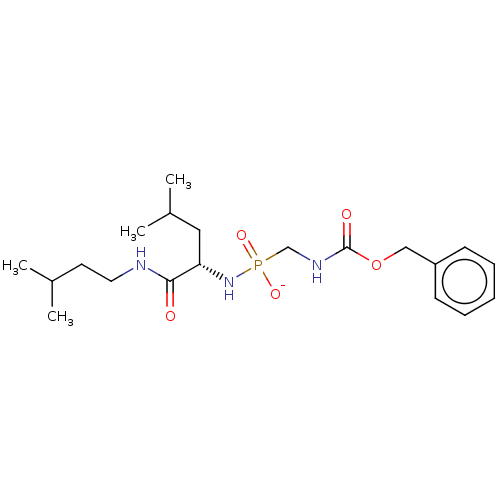 (CHEMBL3409506) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus TLN using 2-furanacryloyl-Gly-Leu-NH2 substrate by biochemical assay | Eur J Med Chem 90: 897-915 (2015) Article DOI: 10.1016/j.ejmech.2014.11.056 BindingDB Entry DOI: 10.7270/Q2FJ2JFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50474120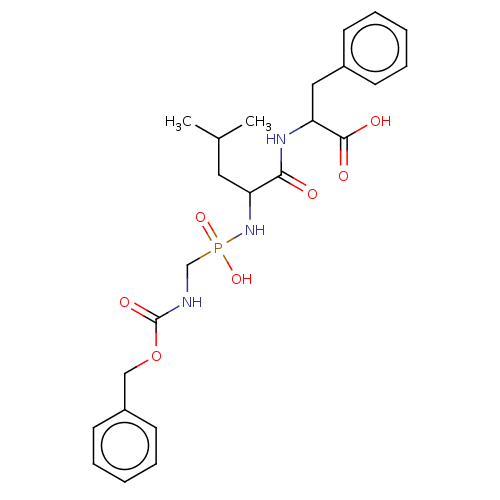 (CHEMBL290692) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 75.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Marburg Curated by ChEMBL | Assay Description pKi value expresses binding affinity against thermolysin pKi = -logKi (where Ki is in mol/L) | J Med Chem 45: 1585-97 (2002) Article DOI: 10.1021/jm011039x BindingDB Entry DOI: 10.7270/Q25B057T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50070932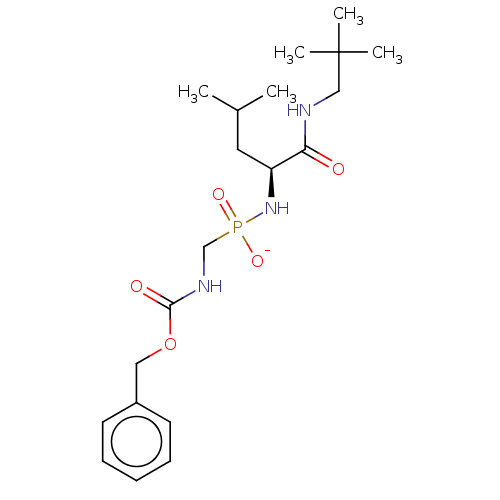 (CHEMBL3409515) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus TLN using 2-furanacryloyl-Gly-Leu-NH2 substrate by biochemical assay | Eur J Med Chem 90: 897-915 (2015) Article DOI: 10.1016/j.ejmech.2014.11.056 BindingDB Entry DOI: 10.7270/Q2FJ2JFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50474139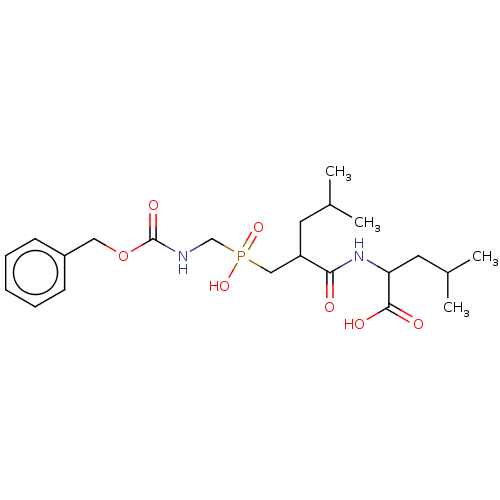 (CHEMBL41376) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 181 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Marburg Curated by ChEMBL | Assay Description pKi value expresses binding affinity against thermolysin pKi = -logKi (where Ki is in mol/L) | J Med Chem 45: 1585-97 (2002) Article DOI: 10.1021/jm011039x BindingDB Entry DOI: 10.7270/Q25B057T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50070928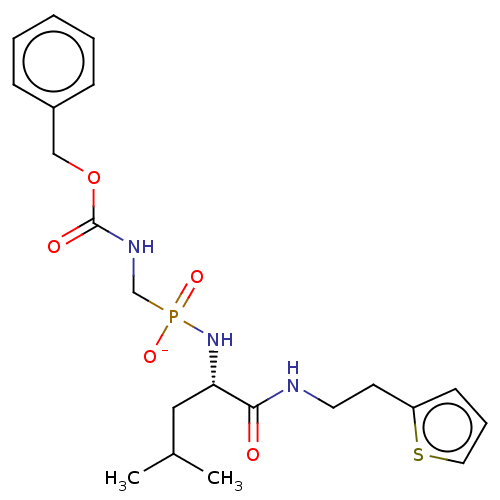 (CHEMBL3409519) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 189 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus TLN using 2-furanacryloyl-Gly-Leu-NH2 substrate by biochemical assay | Eur J Med Chem 90: 897-915 (2015) Article DOI: 10.1016/j.ejmech.2014.11.056 BindingDB Entry DOI: 10.7270/Q2FJ2JFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50474086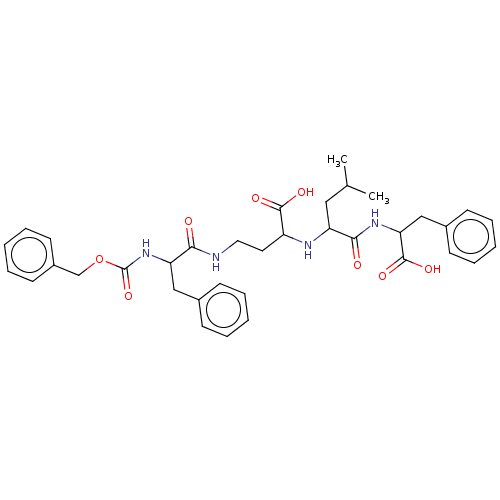 (CHEMBL288359) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 218 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Marburg Curated by ChEMBL | Assay Description pKi value expresses binding affinity against thermolysin pKi = -logKi (where Ki is in mol/L) | J Med Chem 45: 1585-97 (2002) Article DOI: 10.1021/jm011039x BindingDB Entry DOI: 10.7270/Q25B057T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50070943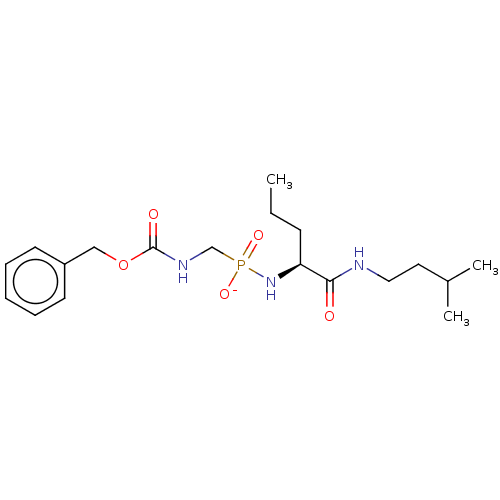 (CHEMBL3409505) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 219 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The State University of New York Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus TLN using 2-furanacryloyl-Gly-Leu-NH2 substrate by biochemical assay | Eur J Med Chem 90: 897-915 (2015) Article DOI: 10.1016/j.ejmech.2014.11.056 BindingDB Entry DOI: 10.7270/Q2FJ2JFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50321123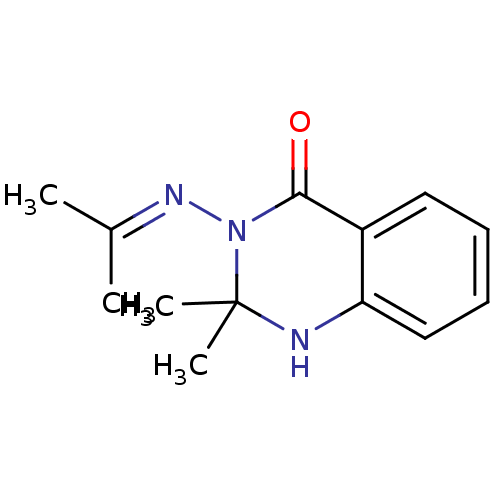 (3-(Isopropylideneamino)-2,2-dimethyl-2,3-dihydroqu...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 243 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Troms£ Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus thermolysin after 15 mins by microplate fluorescence analysis in presence of 0.5 to 2 mM substrate FaGLa | Bioorg Med Chem 18: 4317-27 (2010) Article DOI: 10.1016/j.bmc.2010.04.083 BindingDB Entry DOI: 10.7270/Q2QV3MP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 185 total ) | Next | Last >> |