

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutathione reductase (Saccharomyces cerevisiae) | BDBM50333880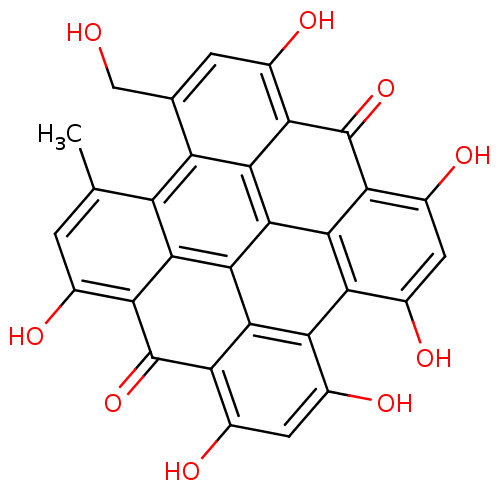 (CHEMBL1614664 | Hypericin | Pseudohypericin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of yeast glutathione reductase by spectrophotometry | Bioorg Med Chem 19: 631-41 (2011) Article DOI: 10.1016/j.bmc.2010.10.045 BindingDB Entry DOI: 10.7270/Q25D8S4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase (Saccharomyces cerevisiae) | BDBM50060874 (1,3,4,6,8,13-hexahydroxy-10,11-dimethylphenanthro[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of yeast glutathione reductase by spectrophotometry | Bioorg Med Chem 19: 631-41 (2011) Article DOI: 10.1016/j.bmc.2010.10.045 BindingDB Entry DOI: 10.7270/Q25D8S4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase (Saccharomyces cerevisiae) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description In vitro inhibitory activity against glutathione reductase (GR) from bakers yeast | J Med Chem 46: 1419-28 (2003) Article DOI: 10.1021/jm030762f BindingDB Entry DOI: 10.7270/Q21G0KN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase (Saccharomyces cerevisiae) | BDBM50171438 (2-Acetylamino-3-[4-(2-acetylamino-2-carboxy-ethyls...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
South Dakota State University Curated by ChEMBL | Assay Description Inhibition of yeast glutathione reductase done for 30 minutes in pH 7.4 at 25 degree C with compound (0.2 mM) | J Med Chem 48: 5224-31 (2005) Article DOI: 10.1021/jm050030i BindingDB Entry DOI: 10.7270/Q2SQ8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase (Saccharomyces cerevisiae) | BDBM50171440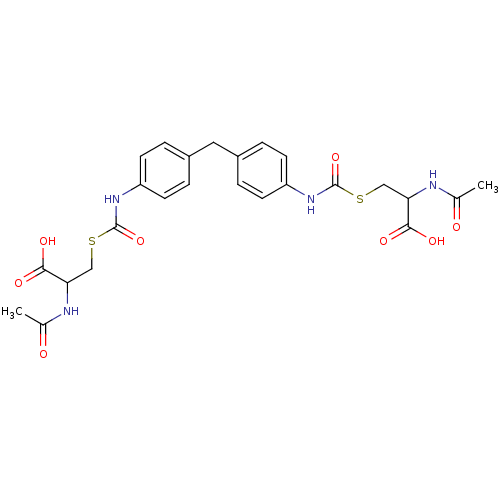 (2-Acetylamino-3-{4-[4-(2-acetylamino-2-carboxy-eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
South Dakota State University Curated by ChEMBL | Assay Description Inhibition of yeast glutathione reductase over 30 minutes pH 7.4 at 25 degree C | J Med Chem 48: 5224-31 (2005) Article DOI: 10.1021/jm050030i BindingDB Entry DOI: 10.7270/Q2SQ8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase (Saccharomyces cerevisiae) | BDBM50171441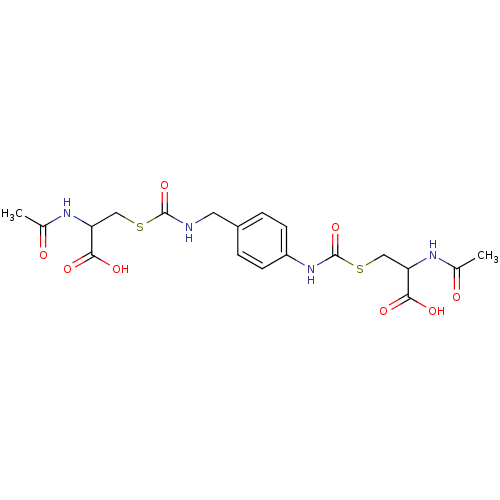 (2-Acetylamino-3-[4-(2-acetylamino-2-carboxy-ethyls...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.17E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
South Dakota State University Curated by ChEMBL | Assay Description Inhibition of yeast glutathione reductase over 30 minutes pH 7.4 at 25 degree C | J Med Chem 48: 5224-31 (2005) Article DOI: 10.1021/jm050030i BindingDB Entry DOI: 10.7270/Q2SQ8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase (Saccharomyces cerevisiae) | BDBM50171444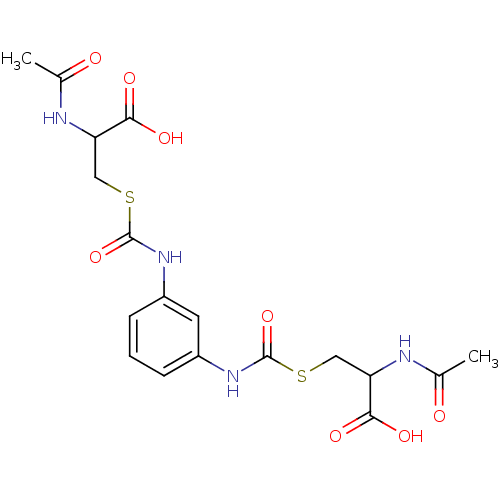 (2-Acetylamino-3-[3-(2-acetylamino-2-carboxy-ethyls...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.49E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
South Dakota State University Curated by ChEMBL | Assay Description Inhibition of yeast glutathione reductase over 30 minutes pH 7.4 at 25 degree C | J Med Chem 48: 5224-31 (2005) Article DOI: 10.1021/jm050030i BindingDB Entry DOI: 10.7270/Q2SQ8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase (Saccharomyces cerevisiae) | BDBM50015950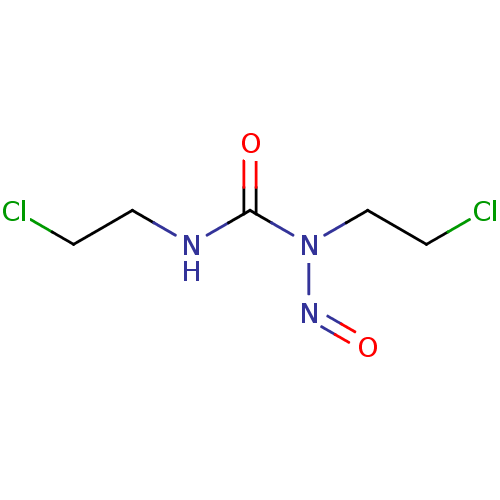 (1,3-bis(2-chloroethyl)-1-nitrosourea | Bicnu (TN) ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.41E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
South Dakota State University Curated by ChEMBL | Assay Description Inhibition of yeast glutathione reductase over 30 minutes pH 7.4 at 25 degree C | J Med Chem 48: 5224-31 (2005) Article DOI: 10.1021/jm050030i BindingDB Entry DOI: 10.7270/Q2SQ8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase (Saccharomyces cerevisiae) | BDBM50171442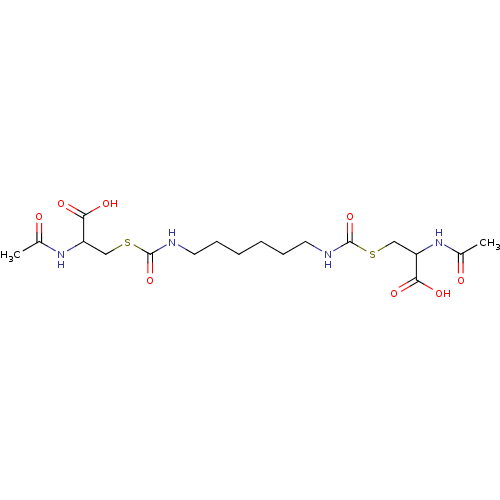 (2-Acetylamino-3-[6-(2-acetylamino-2-carboxy-ethyls...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.55E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
South Dakota State University Curated by ChEMBL | Assay Description Inhibition of yeast glutathione reductase over 30 minutes pH 7.4 at 25 degree C | J Med Chem 48: 5224-31 (2005) Article DOI: 10.1021/jm050030i BindingDB Entry DOI: 10.7270/Q2SQ8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase (Saccharomyces cerevisiae) | BDBM50171443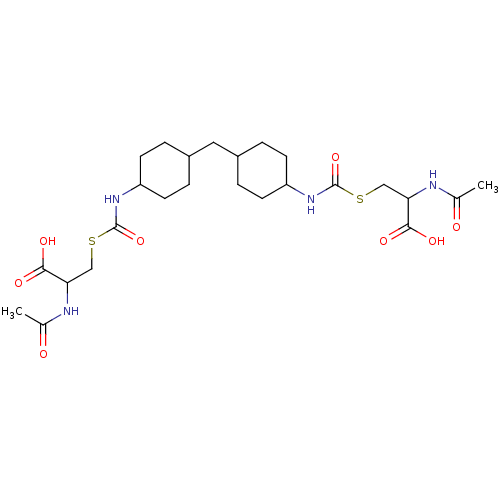 (2-Acetylamino-3-{4-[4-(2-acetylamino-2-carboxy-eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.77E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
South Dakota State University Curated by ChEMBL | Assay Description Inhibition of yeast glutathione reductase over 30 minutes pH 7.4 at 25 degree C | J Med Chem 48: 5224-31 (2005) Article DOI: 10.1021/jm050030i BindingDB Entry DOI: 10.7270/Q2SQ8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase (Saccharomyces cerevisiae) | BDBM50171439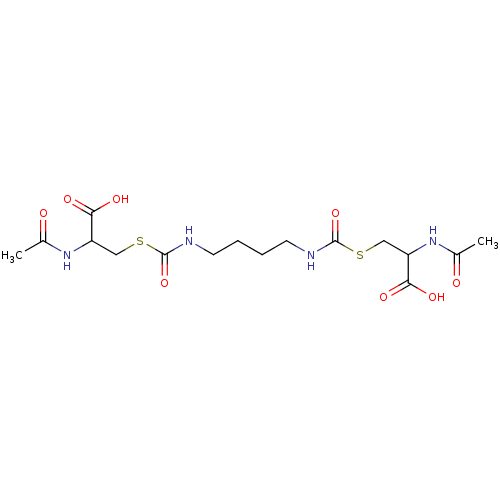 (2-Acetylamino-3-[4-(2-acetylamino-2-carboxy-ethyls...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.15E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
South Dakota State University Curated by ChEMBL | Assay Description Inhibition of yeast glutathione reductase over 30 minutes pH 7.4 at 25 degree C | J Med Chem 48: 5224-31 (2005) Article DOI: 10.1021/jm050030i BindingDB Entry DOI: 10.7270/Q2SQ8ZW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||