

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM468963 (1-[1-[(3R,5S,6R)-5-Amino-6-(2,5-difluorophenyl)tet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.474 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Human DPP-4 ((39-766)-His) and mouse DPP-4 ((29-760)-His) are purified by gel chromatography for use in the assay. The final concentration of hDPP-4 ... | US Patent US10807971 (2020) BindingDB Entry DOI: 10.7270/Q2Z89GGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM468964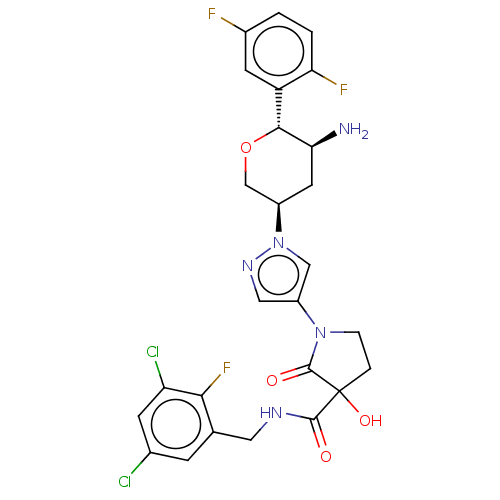 (1-[1-[(3R,5S,6R)-5-Amino-6-(2,5-difluorophenyl)tet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.498 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Human DPP-4 ((39-766)-His) and mouse DPP-4 ((29-760)-His) are purified by gel chromatography for use in the assay. The final concentration of hDPP-4 ... | US Patent US10807971 (2020) BindingDB Entry DOI: 10.7270/Q2Z89GGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM468972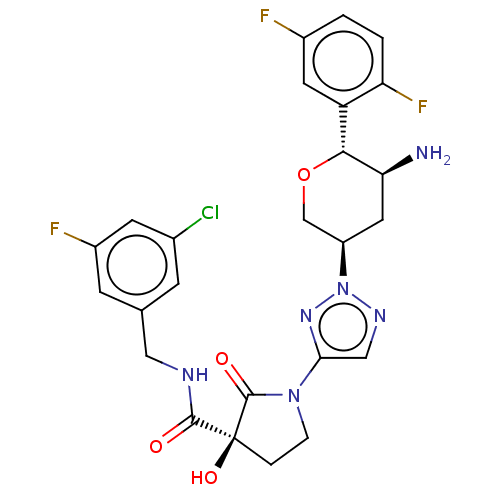 ((3S)-1-[2-[(3R,5S,6R)-5-Amino-6-(2,5-difluoropheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.564 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Human DPP-4 ((39-766)-His) and mouse DPP-4 ((29-760)-His) are purified by gel chromatography for use in the assay. The final concentration of hDPP-4 ... | US Patent US10807971 (2020) BindingDB Entry DOI: 10.7270/Q2Z89GGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50509436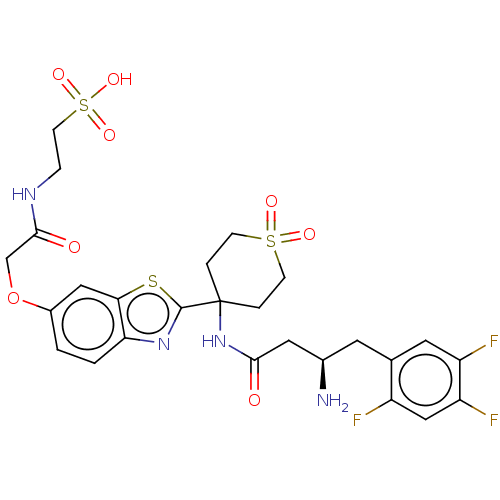 (CHEMBL4463027) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay | J Med Chem 62: 10919-10925 (2019) Article DOI: 10.1021/acs.jmedchem.9b01649 BindingDB Entry DOI: 10.7270/Q2PN98X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50509435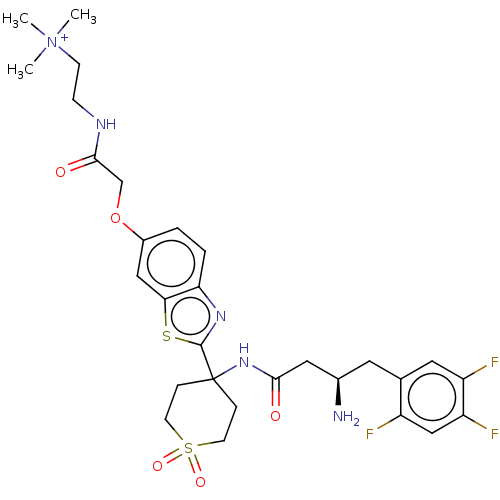 (CHEMBL4593371) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay | J Med Chem 62: 10919-10925 (2019) Article DOI: 10.1021/acs.jmedchem.9b01649 BindingDB Entry DOI: 10.7270/Q2PN98X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM468975 (1-[2-[(3R,5S,6R)-5-Amino-6- (2,5- difluorophenyl)t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.52 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Human DPP-4 ((39-766)-His) and mouse DPP-4 ((29-760)-His) are purified by gel chromatography for use in the assay. The final concentration of hDPP-4 ... | US Patent US10807971 (2020) BindingDB Entry DOI: 10.7270/Q2Z89GGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM468974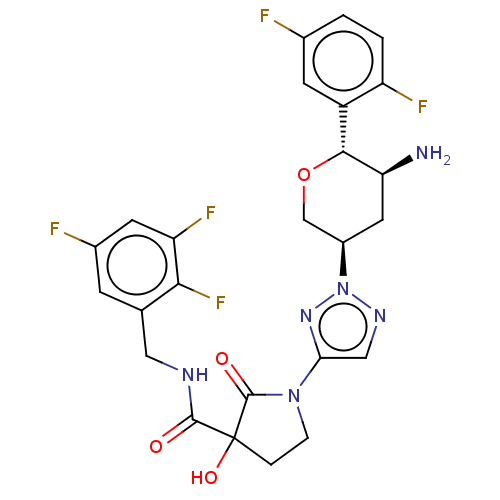 (1-[2-[(3R,5S,6R)-5-Amino-6- (2,5- difluorophenyl)t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.52 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Human DPP-4 ((39-766)-His) and mouse DPP-4 ((29-760)-His) are purified by gel chromatography for use in the assay. The final concentration of hDPP-4 ... | US Patent US10807971 (2020) BindingDB Entry DOI: 10.7270/Q2Z89GGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50509441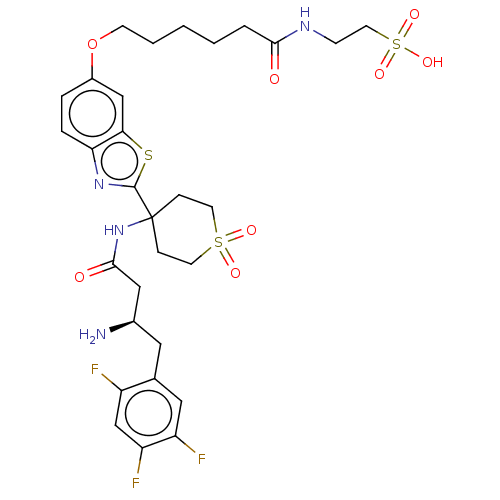 (CHEMBL4452661) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay | J Med Chem 62: 10919-10925 (2019) Article DOI: 10.1021/acs.jmedchem.9b01649 BindingDB Entry DOI: 10.7270/Q2PN98X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM468977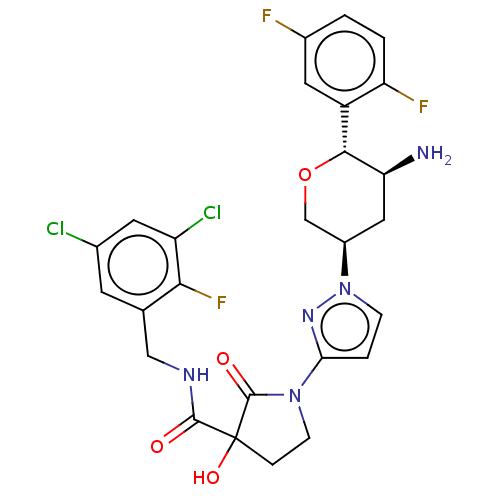 (1-[2-[(3R,5S,6R)-5-Aamino- 6-(2,5- difluorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.03 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Human DPP-4 ((39-766)-His) and mouse DPP-4 ((29-760)-His) are purified by gel chromatography for use in the assay. The final concentration of hDPP-4 ... | US Patent US10807971 (2020) BindingDB Entry DOI: 10.7270/Q2Z89GGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50509440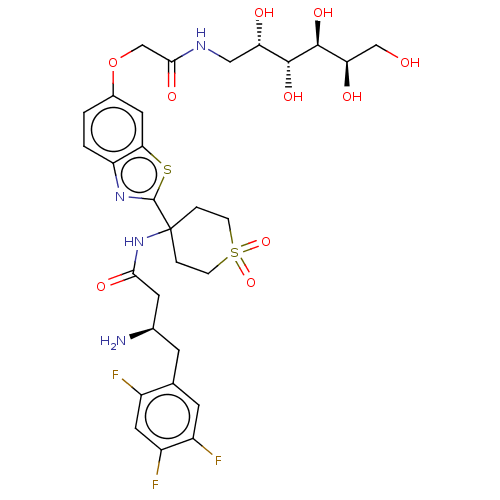 (CHEMBL4533178) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay | J Med Chem 62: 10919-10925 (2019) Article DOI: 10.1021/acs.jmedchem.9b01649 BindingDB Entry DOI: 10.7270/Q2PN98X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50276520 ((2R,4R)-4-fluoro-1-(2-(4-methyl-1-(methylsulfonyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited Curated by ChEMBL | Assay Description Inhibition of mouse DPP4 | Bioorg Med Chem 17: 1783-802 (2009) Article DOI: 10.1016/j.bmc.2009.01.061 BindingDB Entry DOI: 10.7270/Q2VD70DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM468976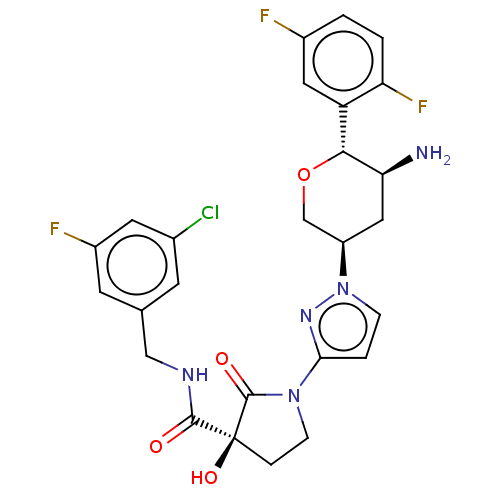 ((3S)-1-[1-[(3R,5S,6R)-5-Amino-6-(2,5-difluoropheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.64 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Human DPP-4 ((39-766)-His) and mouse DPP-4 ((29-760)-His) are purified by gel chromatography for use in the assay. The final concentration of hDPP-4 ... | US Patent US10807971 (2020) BindingDB Entry DOI: 10.7270/Q2Z89GGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50509434 (CHEMBL4443285) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay | J Med Chem 62: 10919-10925 (2019) Article DOI: 10.1021/acs.jmedchem.9b01649 BindingDB Entry DOI: 10.7270/Q2PN98X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50509433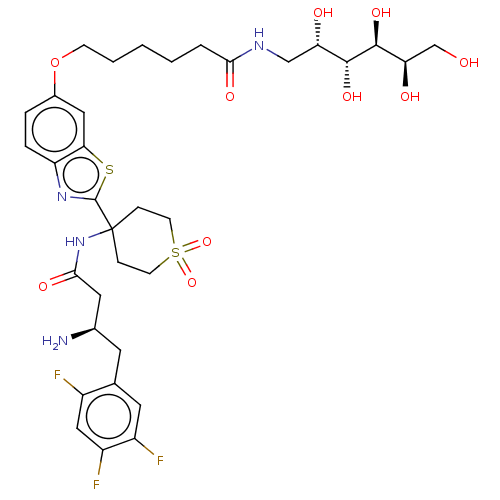 (CHEMBL4532312) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay | J Med Chem 62: 10919-10925 (2019) Article DOI: 10.1021/acs.jmedchem.9b01649 BindingDB Entry DOI: 10.7270/Q2PN98X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM468973 (1-[2-[(3R,5S,6R)-5-Amino-6- (2,5- difluorophenyl)t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.57 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Human DPP-4 ((39-766)-His) and mouse DPP-4 ((29-760)-His) are purified by gel chromatography for use in the assay. The final concentration of hDPP-4 ... | US Patent US10807971 (2020) BindingDB Entry DOI: 10.7270/Q2Z89GGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM468958 (1-[2-[(3R,5S,6R)-5-Amino- 6-(2,5- difluorophenyl)t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.46 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Human DPP-4 ((39-766)-His) and mouse DPP-4 ((29-760)-His) are purified by gel chromatography for use in the assay. The final concentration of hDPP-4 ... | US Patent US10807971 (2020) BindingDB Entry DOI: 10.7270/Q2Z89GGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM468957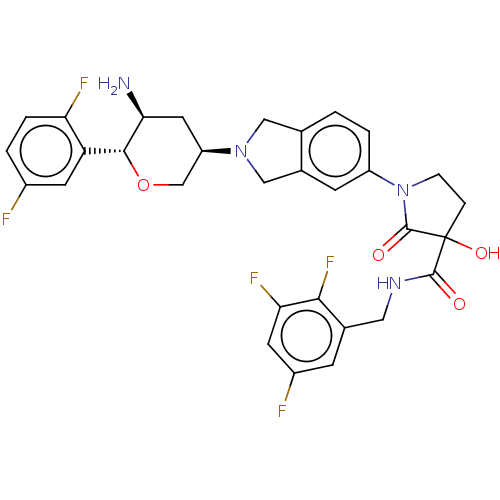 (1-[2-[(3R,5S,6R)-5-Amino-6-(2,5-difluorophenyl)tet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.53 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Human DPP-4 ((39-766)-His) and mouse DPP-4 ((29-760)-His) are purified by gel chromatography for use in the assay. The final concentration of hDPP-4 ... | US Patent US10807971 (2020) BindingDB Entry DOI: 10.7270/Q2Z89GGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM468979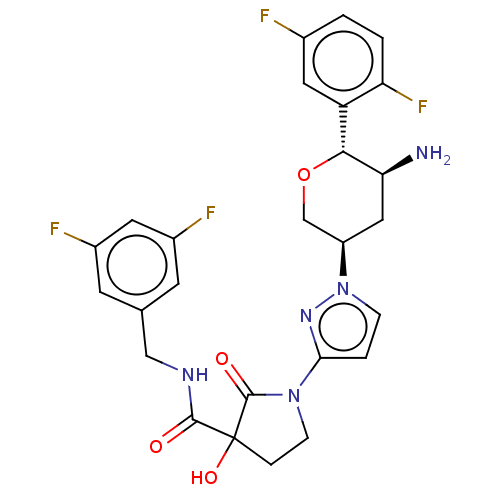 (1-[1-[(3R,5S,6R)-5-Amino- 6-(2,5- difluorophenyl)t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.73 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Human DPP-4 ((39-766)-His) and mouse DPP-4 ((29-760)-His) are purified by gel chromatography for use in the assay. The final concentration of hDPP-4 ... | US Patent US10807971 (2020) BindingDB Entry DOI: 10.7270/Q2Z89GGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50210527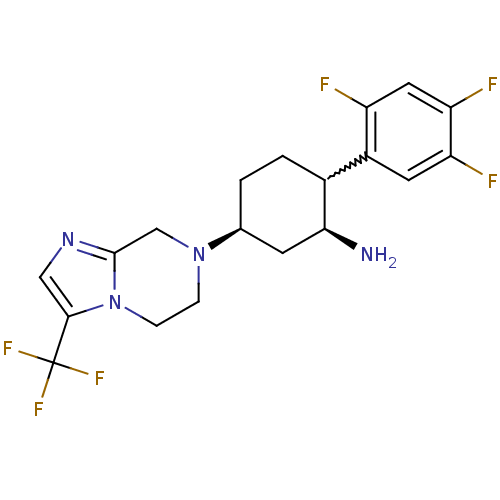 ((1S,2R,5S)-5-(3-(trifluoromethyl)-5,6-dihydroimida...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse DPP4 | Bioorg Med Chem Lett 17: 3384-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.095 BindingDB Entry DOI: 10.7270/Q2NK3DQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50232502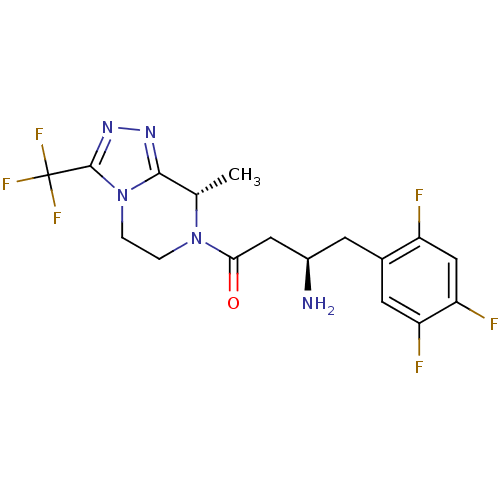 ((2R)-4-[(8S)-8-methyl-3-(trifluoromethyl)-5,6-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse DPP4 at 1 mg/kg | J Med Chem 51: 589-602 (2008) Article DOI: 10.1021/jm070330v BindingDB Entry DOI: 10.7270/Q2B27W4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM468978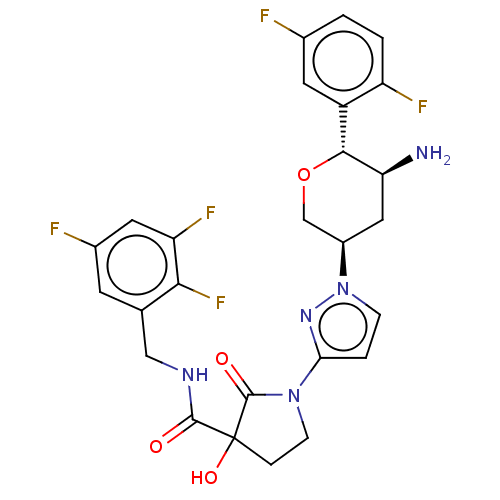 (1-[1-[(3R,5S,6R)-5-Amino-6- (2,5- difluorophenyl)t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Human DPP-4 ((39-766)-His) and mouse DPP-4 ((29-760)-His) are purified by gel chromatography for use in the assay. The final concentration of hDPP-4 ... | US Patent US10807971 (2020) BindingDB Entry DOI: 10.7270/Q2Z89GGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay | J Med Chem 62: 10919-10925 (2019) Article DOI: 10.1021/acs.jmedchem.9b01649 BindingDB Entry DOI: 10.7270/Q2PN98X6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM468980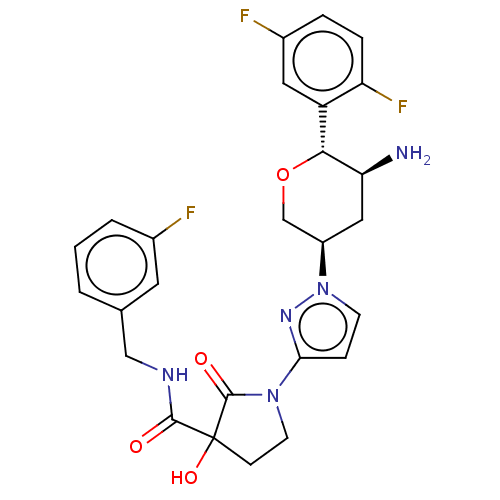 (1-[1-[(3R,5S,6R)-5-Amino- 6-(2,5- difluorophenyl)t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Human DPP-4 ((39-766)-His) and mouse DPP-4 ((29-760)-His) are purified by gel chromatography for use in the assay. The final concentration of hDPP-4 ... | US Patent US10807971 (2020) BindingDB Entry DOI: 10.7270/Q2Z89GGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM468981 ((3S)-1-[2-[1-[(3R,5S,6R)-5-Amino-6-(2,5-difluoroph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Human DPP-4 ((39-766)-His) and mouse DPP-4 ((29-760)-His) are purified by gel chromatography for use in the assay. The final concentration of hDPP-4 ... | US Patent US10807971 (2020) BindingDB Entry DOI: 10.7270/Q2Z89GGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM468961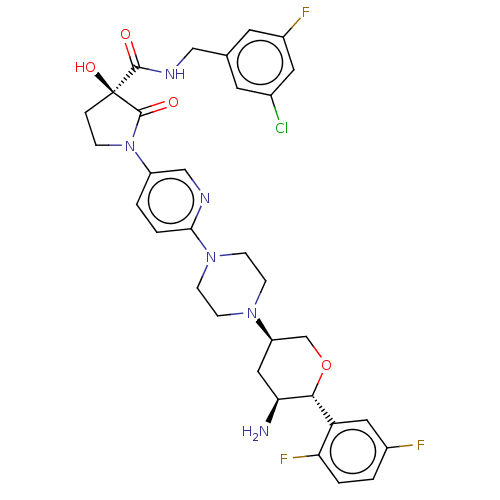 ((3S)-1-[6-[4-[(3S,5S,6R)-5-Amino-6-(2,5-difluoroph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Human DPP-4 ((39-766)-His) and mouse DPP-4 ((29-760)-His) are purified by gel chromatography for use in the assay. The final concentration of hDPP-4 ... | US Patent US10807971 (2020) BindingDB Entry DOI: 10.7270/Q2Z89GGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM468960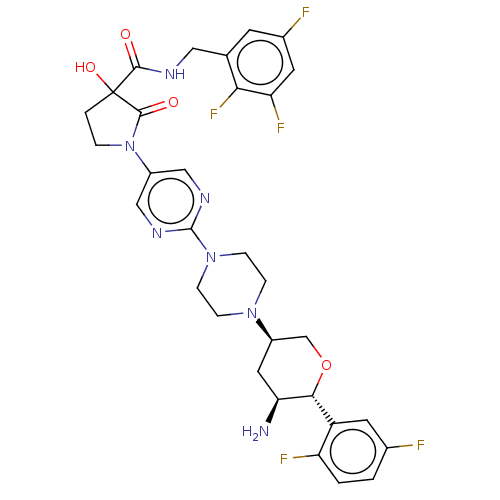 (1-[2-[4-[(3R,5S,6R)-5- Amino-6-(2,5- difluoropheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Human DPP-4 ((39-766)-His) and mouse DPP-4 ((29-760)-His) are purified by gel chromatography for use in the assay. The final concentration of hDPP-4 ... | US Patent US10807971 (2020) BindingDB Entry DOI: 10.7270/Q2Z89GGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM468959 (1-[2-[4-[(3R,5S,6R)-5-Amino-6-(2,5-difluorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description Human DPP-4 ((39-766)-His) and mouse DPP-4 ((29-760)-His) are purified by gel chromatography for use in the assay. The final concentration of hDPP-4 ... | US Patent US10807971 (2020) BindingDB Entry DOI: 10.7270/Q2Z89GGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50003020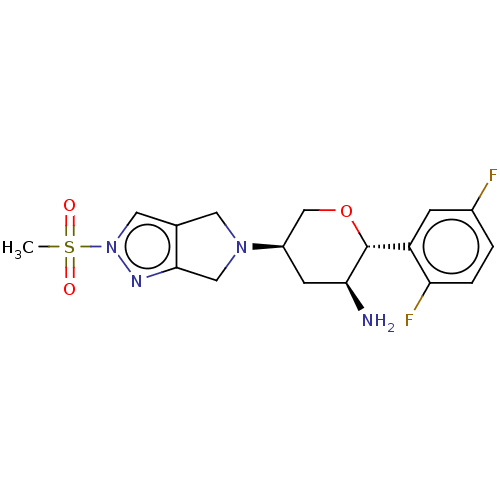 (MK-3102 | OMARIGLIPTIN | US10155775, Omarigliptin ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of DPP4 in mouse plasma | J Med Chem 57: 3205-12 (2014) Article DOI: 10.1021/jm401992e BindingDB Entry DOI: 10.7270/Q2WD423H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50296490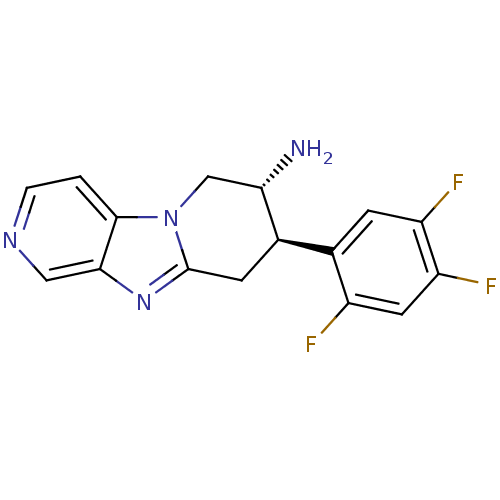 ((7R,8R)-8-(2,4,5-Trifluoro-phenyl)-6,7,8,9-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Inhibition of C57BL/6N mouse DPP4 | Bioorg Med Chem Lett 19: 4097-101 (2009) Article DOI: 10.1016/j.bmcl.2009.06.011 BindingDB Entry DOI: 10.7270/Q2513Z7C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | Bioorg Med Chem Lett 27: 2313-2318 (2017) Article DOI: 10.1016/j.bmcl.2017.04.036 BindingDB Entry DOI: 10.7270/Q2ZC858S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse DPP4 at 3 mg/kg | J Med Chem 51: 589-602 (2008) Article DOI: 10.1021/jm070330v BindingDB Entry DOI: 10.7270/Q2B27W4M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50509437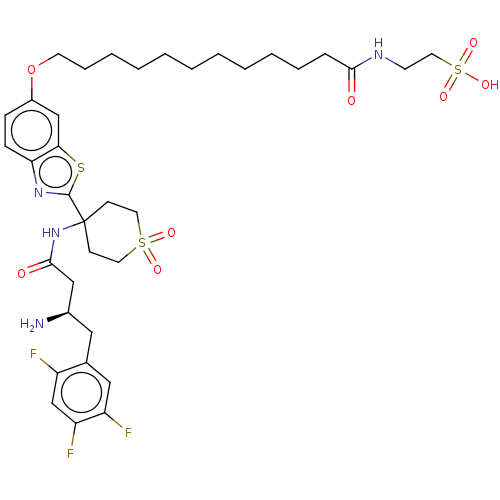 (CHEMBL4445546) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay | J Med Chem 62: 10919-10925 (2019) Article DOI: 10.1021/acs.jmedchem.9b01649 BindingDB Entry DOI: 10.7270/Q2PN98X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50390183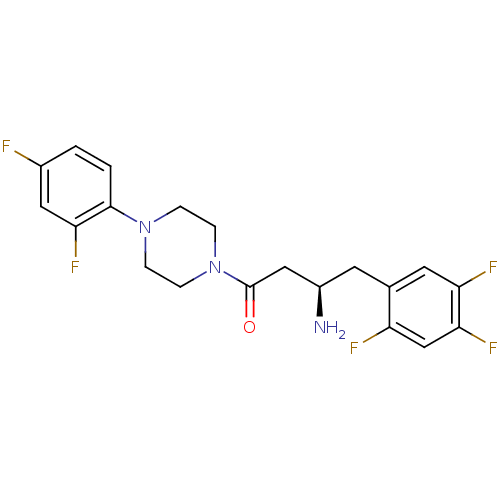 (CHEMBL2069830) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 94.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mouse DPP4 | Bioorg Med Chem Lett 22: 5545-9 (2012) Article DOI: 10.1016/j.bmcl.2012.07.019 BindingDB Entry DOI: 10.7270/Q2K64K49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50509439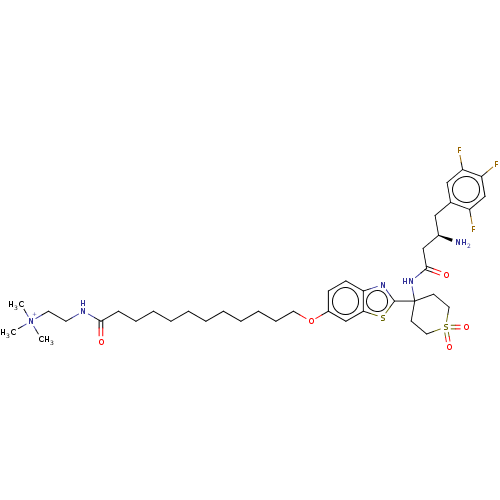 (CHEMBL4442618) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay | J Med Chem 62: 10919-10925 (2019) Article DOI: 10.1021/acs.jmedchem.9b01649 BindingDB Entry DOI: 10.7270/Q2PN98X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50509438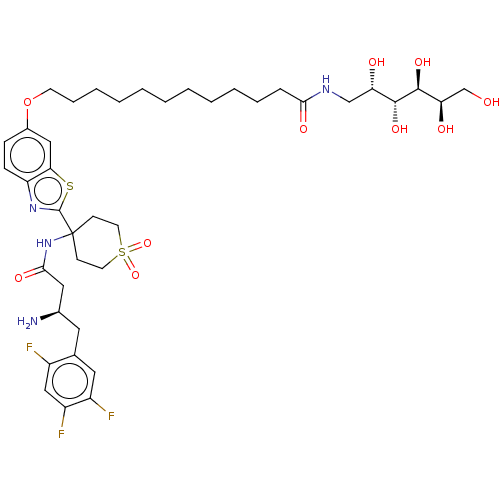 (CHEMBL4584732) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 168 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay | J Med Chem 62: 10919-10925 (2019) Article DOI: 10.1021/acs.jmedchem.9b01649 BindingDB Entry DOI: 10.7270/Q2PN98X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50242266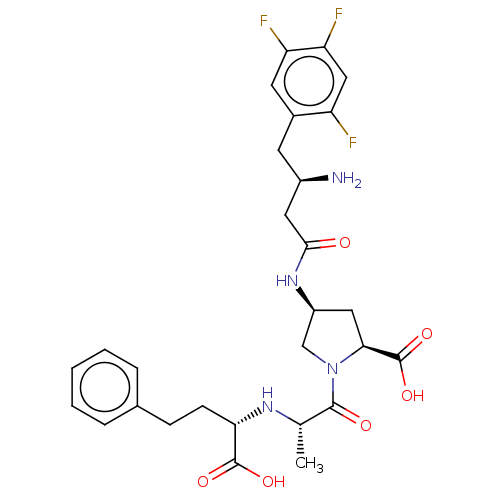 (CHEMBL4067185) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited Curated by ChEMBL | Assay Description Inhibition of ob/ob mouse plasma DPP4 | Bioorg Med Chem Lett 27: 2313-2318 (2017) Article DOI: 10.1016/j.bmcl.2017.04.036 BindingDB Entry DOI: 10.7270/Q2ZC858S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50242264 (CHEMBL4086264) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 488 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited Curated by ChEMBL | Assay Description Inhibition of ob/ob mouse plasma DPP4 | Bioorg Med Chem Lett 27: 2313-2318 (2017) Article DOI: 10.1016/j.bmcl.2017.04.036 BindingDB Entry DOI: 10.7270/Q2ZC858S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50009366 (CHEMBL3233842 | US11504364, Compound Ref.-Comp. | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description DPPIV enzymatic activity assay. To assay baseline dipeptidyl peptidase-4 (DPPIV) activity, 40 ng of recombinant human DPPIV (rhDPPIV) (R&S system, #1... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XS607B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50242265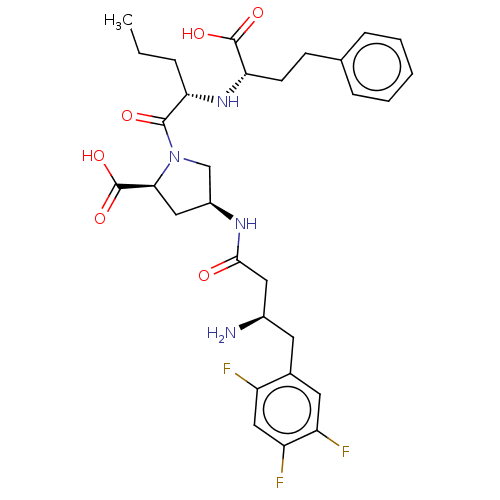 (CHEMBL4078112) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited Curated by ChEMBL | Assay Description Inhibition of ob/ob mouse plasma DPP4 | Bioorg Med Chem Lett 27: 2313-2318 (2017) Article DOI: 10.1016/j.bmcl.2017.04.036 BindingDB Entry DOI: 10.7270/Q2ZC858S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50242274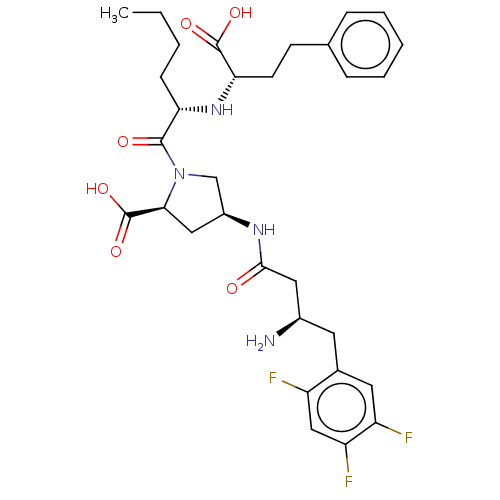 (CHEMBL4090635) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited Curated by ChEMBL | Assay Description Inhibition of ob/ob mouse plasma DPP4 | Bioorg Med Chem Lett 27: 2313-2318 (2017) Article DOI: 10.1016/j.bmcl.2017.04.036 BindingDB Entry DOI: 10.7270/Q2ZC858S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description DPPIV enzymatic activity assay. To assay baseline dipeptidyl peptidase-4 (DPPIV) activity, 40 ng of recombinant human DPPIV (rhDPPIV) (R&S system, #1... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XS607B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM581063 (US11504364, Compound 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description DPPIV enzymatic activity assay. To assay baseline dipeptidyl peptidase-4 (DPPIV) activity, 40 ng of recombinant human DPPIV (rhDPPIV) (R&S system, #1... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XS607B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50614537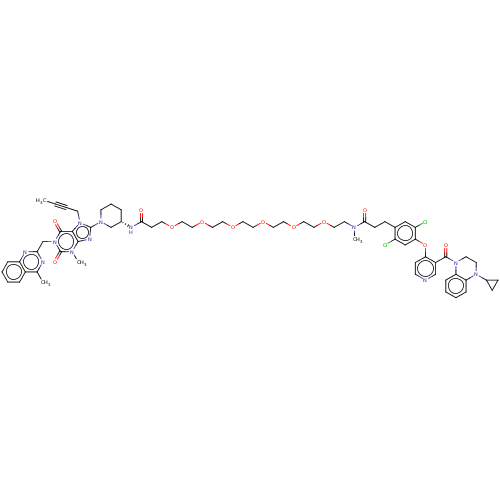 (CHEMBL5275304) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | UniChem | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||