

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50238115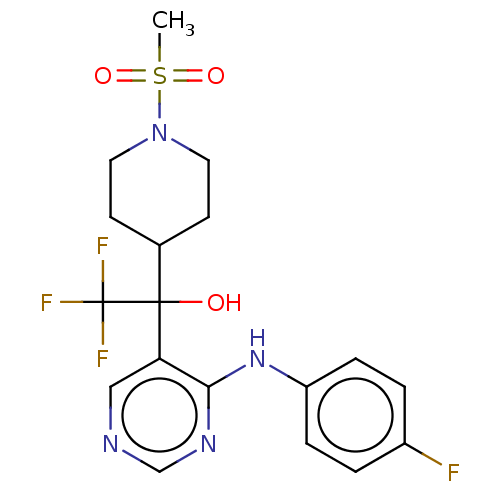 (CHEMBL4082403) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... | Bioorg Med Chem Lett 27: 1902-1906 (2017) Article DOI: 10.1016/j.bmcl.2017.03.034 BindingDB Entry DOI: 10.7270/Q2KS6TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50238115 (CHEMBL4082403) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... | Bioorg Med Chem Lett 27: 1902-1906 (2017) Article DOI: 10.1016/j.bmcl.2017.03.034 BindingDB Entry DOI: 10.7270/Q2KS6TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50238114 (CHEMBL4093575) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% | Bioorg Med Chem Lett 27: 1902-1906 (2017) Article DOI: 10.1016/j.bmcl.2017.03.034 BindingDB Entry DOI: 10.7270/Q2KS6TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50238115 (CHEMBL4082403) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... | Bioorg Med Chem Lett 27: 1902-1906 (2017) Article DOI: 10.1016/j.bmcl.2017.03.034 BindingDB Entry DOI: 10.7270/Q2KS6TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50238103 (CHEMBL4070323) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% | Bioorg Med Chem Lett 27: 1902-1906 (2017) Article DOI: 10.1016/j.bmcl.2017.03.034 BindingDB Entry DOI: 10.7270/Q2KS6TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50238104 (CHEMBL4072871) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... | Bioorg Med Chem Lett 27: 1902-1906 (2017) Article DOI: 10.1016/j.bmcl.2017.03.034 BindingDB Entry DOI: 10.7270/Q2KS6TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of mouse CYP11B2 expressed in renal leiomyoblastoma cells | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50444549 (CHEMBL3099695) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of mouse CYP11B2 expressed in renal leiomyoblastoma cells | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50238110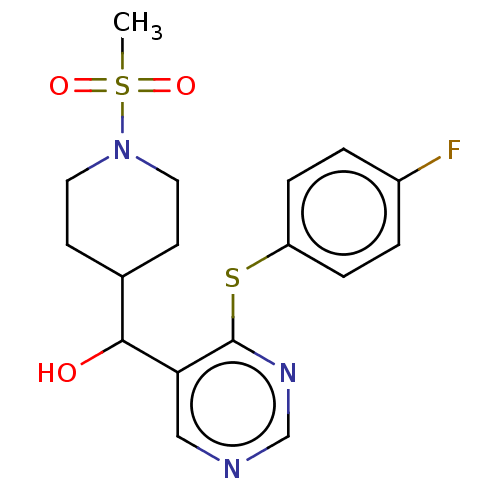 (CHEMBL4088766) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 257 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... | Bioorg Med Chem Lett 27: 1902-1906 (2017) Article DOI: 10.1016/j.bmcl.2017.03.034 BindingDB Entry DOI: 10.7270/Q2KS6TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50238105 (CHEMBL4099824) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... | Bioorg Med Chem Lett 27: 1902-1906 (2017) Article DOI: 10.1016/j.bmcl.2017.03.034 BindingDB Entry DOI: 10.7270/Q2KS6TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50238102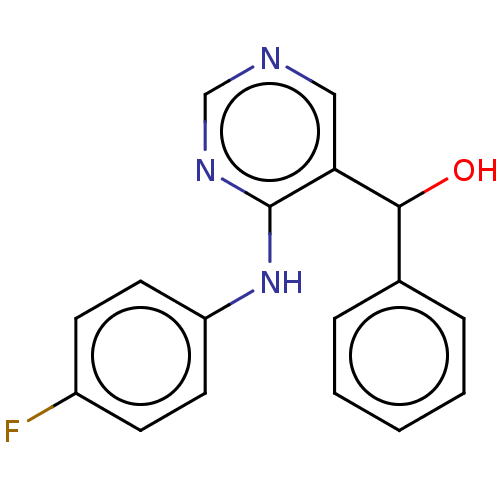 (CHEMBL4065476) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% | Bioorg Med Chem Lett 27: 1902-1906 (2017) Article DOI: 10.1016/j.bmcl.2017.03.034 BindingDB Entry DOI: 10.7270/Q2KS6TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50125049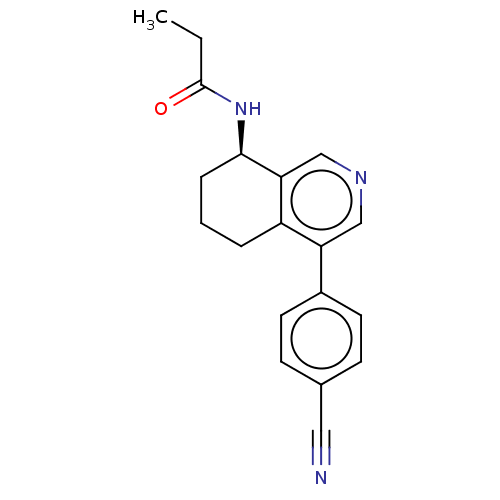 (CHEMBL3623830) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of mouse CYP11B2 expressed in human renal leiomyoblastoma cells using 11-deoxycorticosterone as substrate assessed as formation of aldoste... | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50238109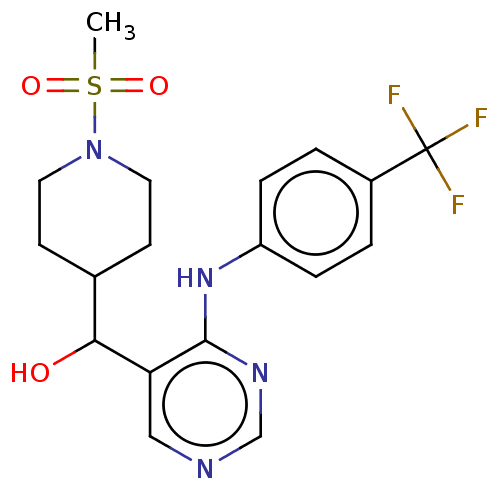 (CHEMBL4091167) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 798 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... | Bioorg Med Chem Lett 27: 1902-1906 (2017) Article DOI: 10.1016/j.bmcl.2017.03.034 BindingDB Entry DOI: 10.7270/Q2KS6TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50238101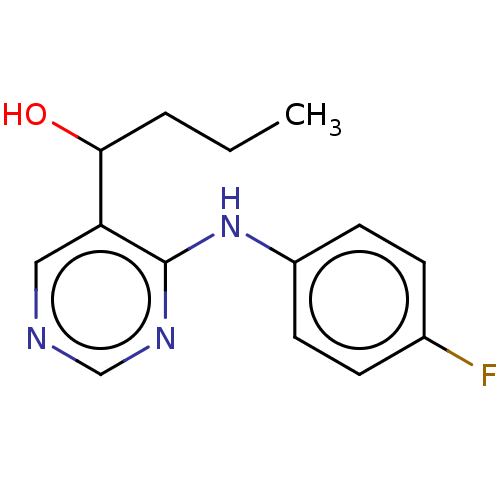 (CHEMBL4104118) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 911 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... | Bioorg Med Chem Lett 27: 1902-1906 (2017) Article DOI: 10.1016/j.bmcl.2017.03.034 BindingDB Entry DOI: 10.7270/Q2KS6TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50238106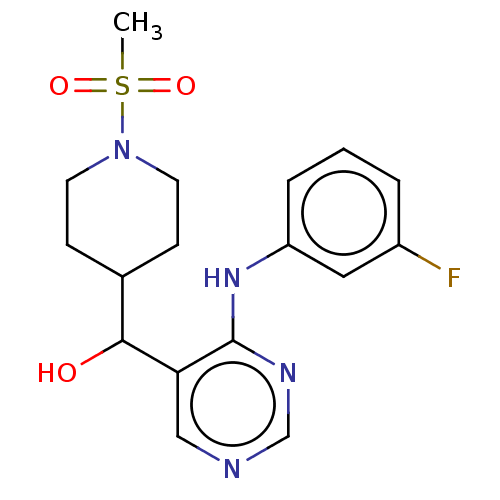 (CHEMBL4072943) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... | Bioorg Med Chem Lett 27: 1902-1906 (2017) Article DOI: 10.1016/j.bmcl.2017.03.034 BindingDB Entry DOI: 10.7270/Q2KS6TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50238121 (CHEMBL4077051) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... | Bioorg Med Chem Lett 27: 1902-1906 (2017) Article DOI: 10.1016/j.bmcl.2017.03.034 BindingDB Entry DOI: 10.7270/Q2KS6TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50238108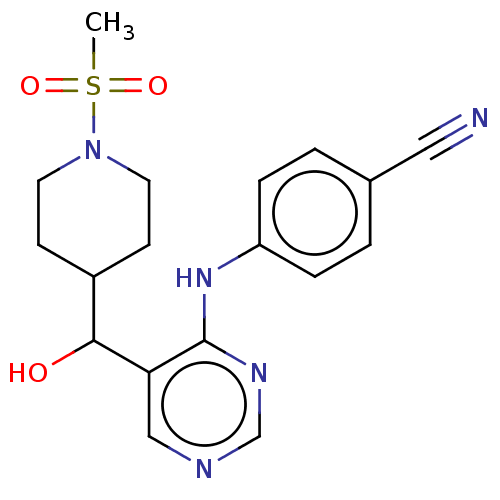 (CHEMBL4070230) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... | Bioorg Med Chem Lett 27: 1902-1906 (2017) Article DOI: 10.1016/j.bmcl.2017.03.034 BindingDB Entry DOI: 10.7270/Q2KS6TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50238111 (CHEMBL4060559) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% | Bioorg Med Chem Lett 27: 1902-1906 (2017) Article DOI: 10.1016/j.bmcl.2017.03.034 BindingDB Entry DOI: 10.7270/Q2KS6TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50238119 (CHEMBL4075207) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... | Bioorg Med Chem Lett 27: 1902-1906 (2017) Article DOI: 10.1016/j.bmcl.2017.03.034 BindingDB Entry DOI: 10.7270/Q2KS6TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50238116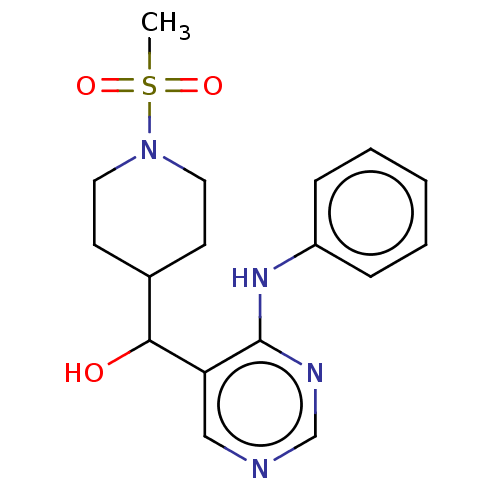 (CHEMBL4079006) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... | Bioorg Med Chem Lett 27: 1902-1906 (2017) Article DOI: 10.1016/j.bmcl.2017.03.034 BindingDB Entry DOI: 10.7270/Q2KS6TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50238117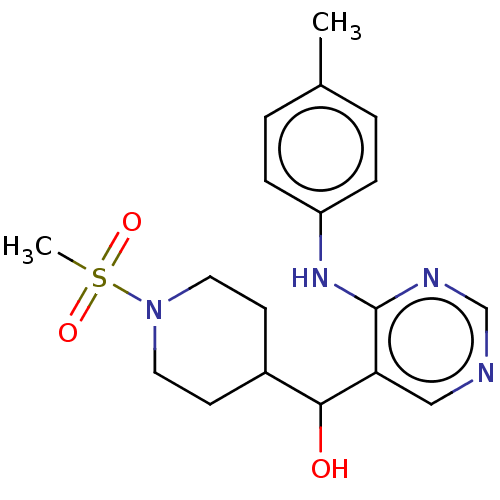 (CHEMBL4092081) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% | Bioorg Med Chem Lett 27: 1902-1906 (2017) Article DOI: 10.1016/j.bmcl.2017.03.034 BindingDB Entry DOI: 10.7270/Q2KS6TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50047262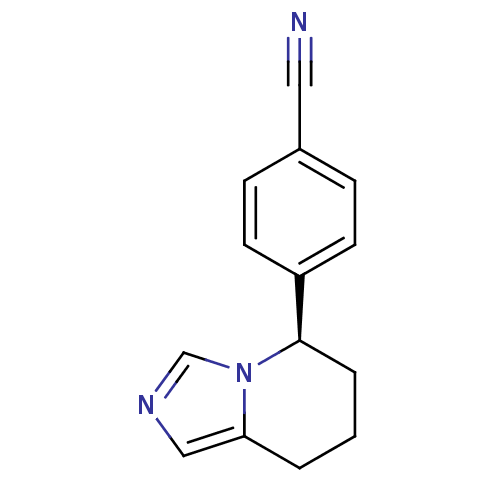 ((R)-4-(5,6,7,8-tetrahydroimidazo[1,5-a]pyridin-5-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% | Bioorg Med Chem Lett 27: 1902-1906 (2017) Article DOI: 10.1016/j.bmcl.2017.03.034 BindingDB Entry DOI: 10.7270/Q2KS6TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50238123 (CHEMBL4061565) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... | Bioorg Med Chem Lett 27: 1902-1906 (2017) Article DOI: 10.1016/j.bmcl.2017.03.034 BindingDB Entry DOI: 10.7270/Q2KS6TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50238112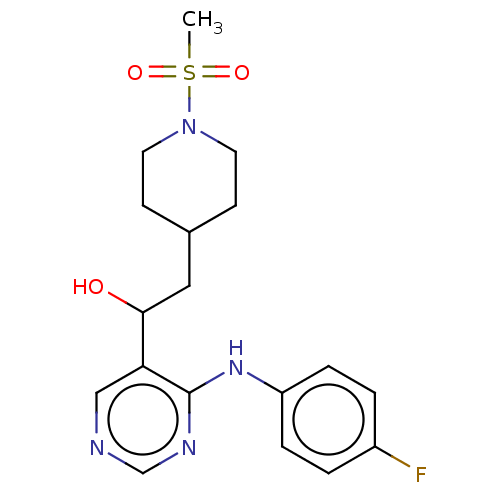 (CHEMBL4101118) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% | Bioorg Med Chem Lett 27: 1902-1906 (2017) Article DOI: 10.1016/j.bmcl.2017.03.034 BindingDB Entry DOI: 10.7270/Q2KS6TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50238113 (CHEMBL4067686) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% | Bioorg Med Chem Lett 27: 1902-1906 (2017) Article DOI: 10.1016/j.bmcl.2017.03.034 BindingDB Entry DOI: 10.7270/Q2KS6TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50238107 (CHEMBL4064367) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... | Bioorg Med Chem Lett 27: 1902-1906 (2017) Article DOI: 10.1016/j.bmcl.2017.03.034 BindingDB Entry DOI: 10.7270/Q2KS6TTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Mus musculus) | BDBM50125050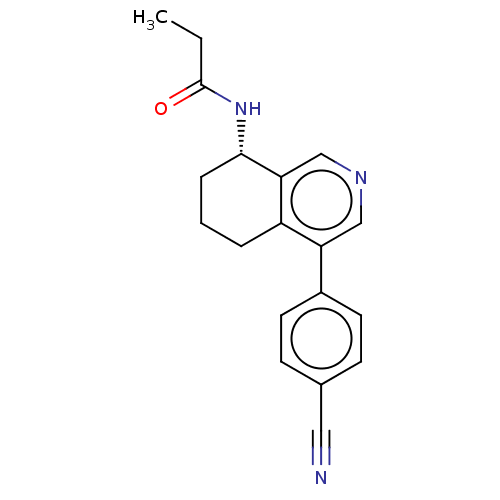 (CHEMBL3623831) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of mouse CYP11B2 expressed in human renal leiomyoblastoma cells using 11-deoxycorticosterone as substrate assessed as formation of aldoste... | J Med Chem 58: 8054-65 (2015) Article DOI: 10.1021/acs.jmedchem.5b00851 BindingDB Entry DOI: 10.7270/Q2K0763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||