

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase (Proteus mirabilis) | BDBM50212643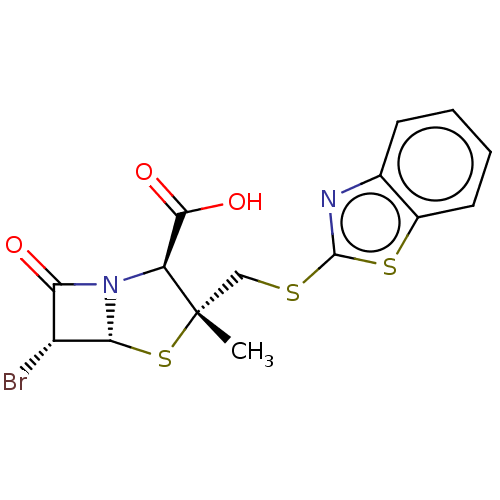 (CHEMBL308516) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | n/a | n/a | 2.25 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Proteus vulgaris HJ33C Class-I beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50212642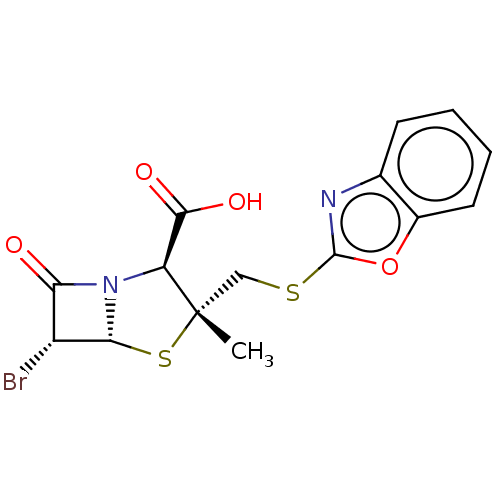 (CHEMBL70269) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 2.33 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Tested for the inhibitory activity against Proteus vulgaris HJ33C Class-I beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50212641 (Brobactam) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | n/a | n/a | 3.57 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Proteus vulgaris HJ33C Class-I beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50212634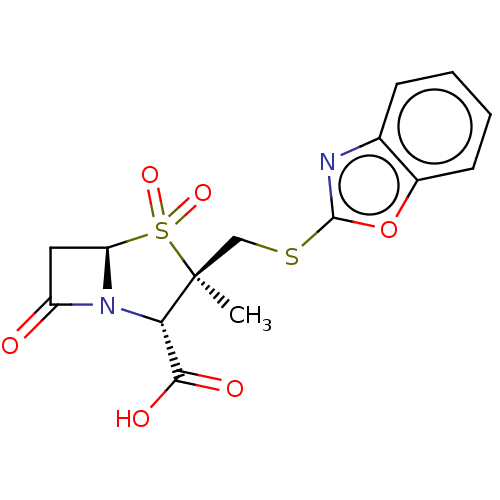 (CHEMBL309009) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 5.23 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Proteus vulgaris HJ33C Class-I beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50212636 (CHEMBL310221) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 7.55 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Proteus vulgaris HJ33C Class-I beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50212635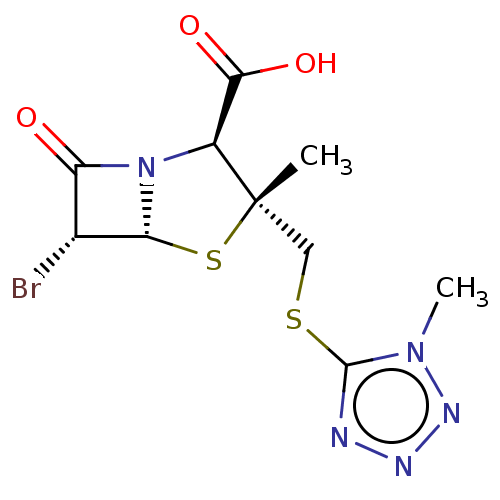 (CHEMBL415266) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 7.61 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Proteus vulgaris HJ33C Class-I beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50212645 (CHEMBL305908) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 7.91 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Proteus vulgaris HJ33C Class-I beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50053173 ((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Proteus vulgaris HJ33C Class-I beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50212646 (CHEMBL72972) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Proteus vulgaris HJ33C Class-I beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50212638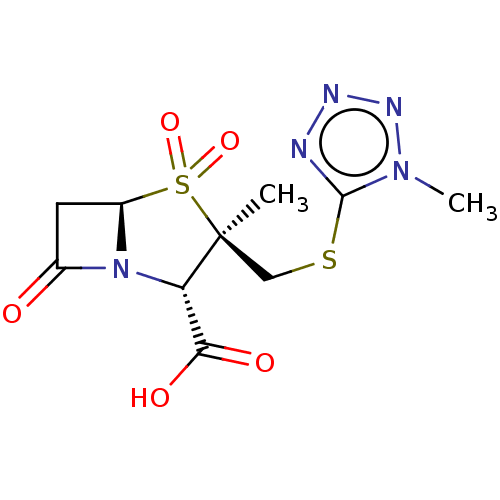 (CHEMBL302512) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Proteus vulgaris HJ33C Class-I beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50212644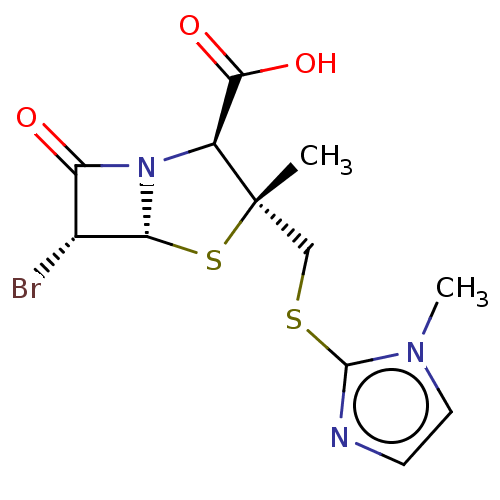 (CHEMBL73450) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 25.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Proteus vulgaris HJ33C Class-I beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50212637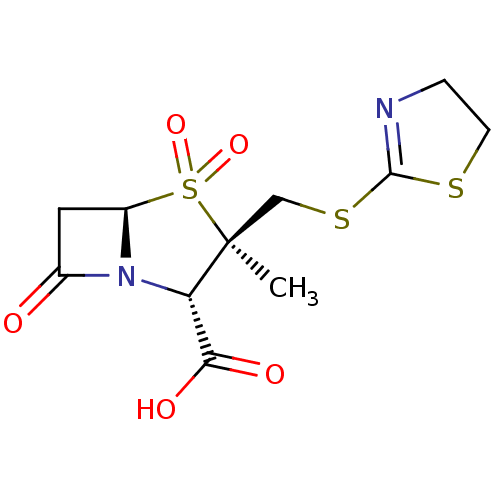 (CHEMBL72046) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 37.1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Proteus vulgaris HJ33C Class-I beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50212640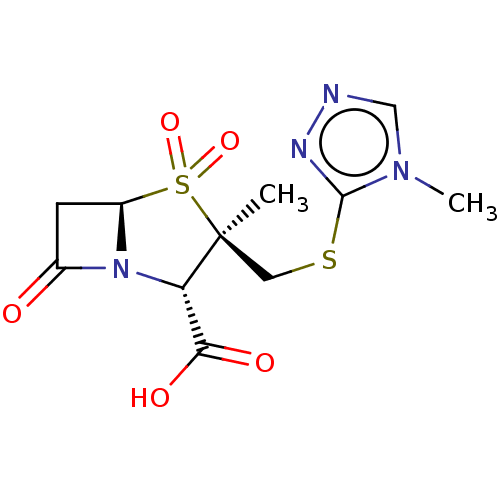 (CHEMBL423309) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 37.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Proteus vulgaris HJ33C Class-I beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 65.3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Proteus vulgaris HJ33C Class-I beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50212639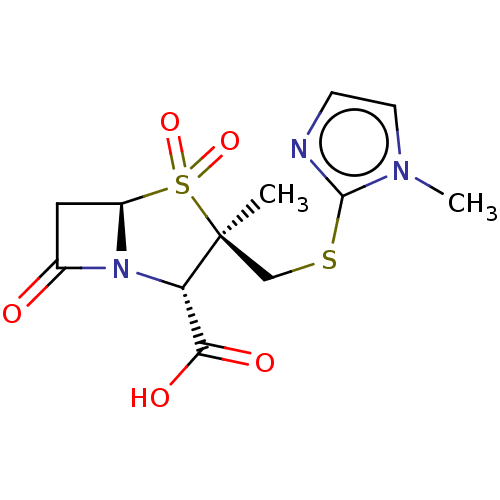 (CHEMBL302241) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 72.4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Proteus vulgaris HJ33C Class-I beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 95.4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Proteus mirabilis C889 beta-lactamase assessed as inhibition of nitrocefin hydrolysis preincubated for 5 mins by microtiter plate assay | J Nat Prod 57: 654-7 (1994) Article DOI: 10.1021/np50107a016 BindingDB Entry DOI: 10.7270/Q2SQ935W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50021954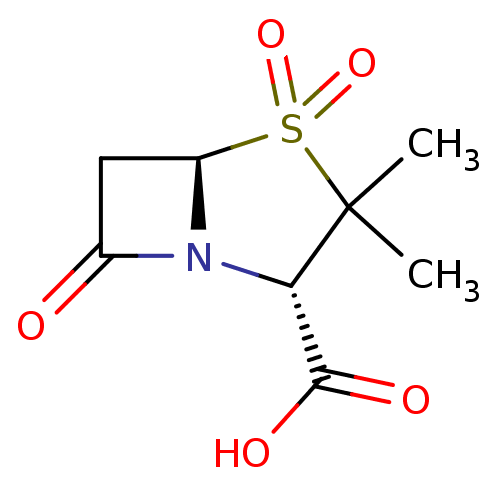 ((2S,5R)-3,3-Dimethyl-4,4,7-trioxo-4lambda*6*-thia-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 342 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Proteus vulgaris HJ33C Class-I beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 9.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Concentration required for its inhibitory activity against Proteus mirabilis C889 class A beta-lactamase | Citation and Details Article DOI: 10.1016/S0960-894X(01)80506-0 BindingDB Entry DOI: 10.7270/Q21V5HPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50469657 (CHEMBL33995 | SB-202742) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.99E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Proteus mirabilis C889 beta-lactamase assessed as inhibition of nitrocefin hydrolysis preincubated for 5 mins by microtiter plate assay | J Nat Prod 57: 654-7 (1994) Article DOI: 10.1021/np50107a016 BindingDB Entry DOI: 10.7270/Q2SQ935W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50469666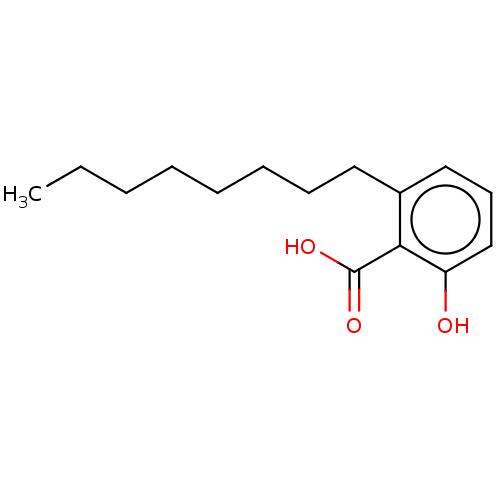 (6-Octylsalicylic Acid | CHEMBL33379) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article | n/a | n/a | 1.55E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Concentration required for its inhibitory activity against Proteus mirabilis C889 class A beta-lactamase | Citation and Details Article DOI: 10.1016/S0960-894X(01)80506-0 BindingDB Entry DOI: 10.7270/Q21V5HPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50469665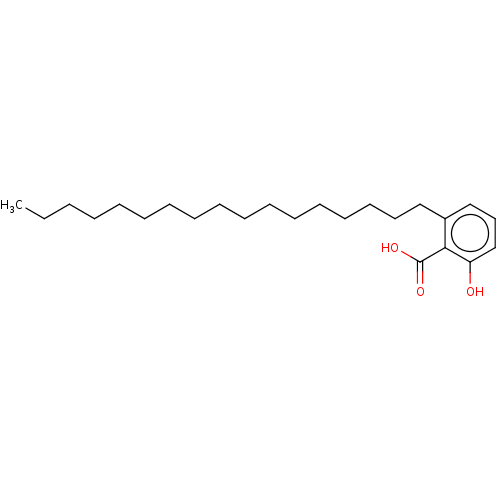 (CHEMBL30914) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article | n/a | n/a | 2.20E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Concentration required for its inhibitory activity against Proteus mirabilis C889 class A beta-lactamase | Citation and Details Article DOI: 10.1016/S0960-894X(01)80506-0 BindingDB Entry DOI: 10.7270/Q21V5HPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50469660 (CHEMBL30915) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article | n/a | n/a | 6.06E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Concentration required for its inhibitory activity against Proteus mirabilis C889 class A beta-lactamase | Citation and Details Article DOI: 10.1016/S0960-894X(01)80506-0 BindingDB Entry DOI: 10.7270/Q21V5HPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50292429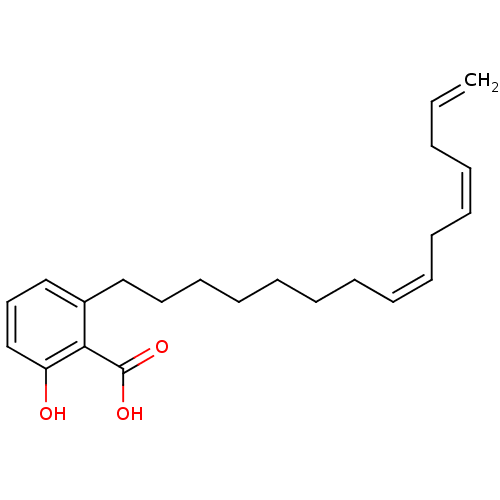 (2-Hydroxy-6-pentadecyl-benzoic acid | 2-Pentadecyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9.05E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Concentration required for its inhibitory activity against Proteus mirabilis C889 class A beta-lactamase | Citation and Details Article DOI: 10.1016/S0960-894X(01)80506-0 BindingDB Entry DOI: 10.7270/Q21V5HPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50469657 (CHEMBL33995 | SB-202742) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article | n/a | n/a | 2.96E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Concentration required for its inhibitory activity against Proteus mirabilis C889 class A beta-lactamase | Citation and Details Article DOI: 10.1016/S0960-894X(01)80506-0 BindingDB Entry DOI: 10.7270/Q21V5HPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50469661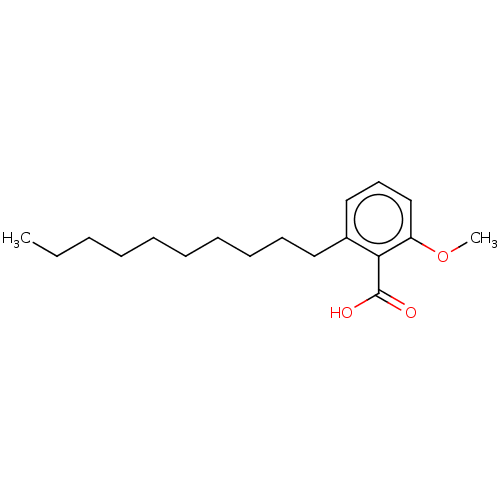 (CHEMBL30870) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article | n/a | n/a | 6.15E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Concentration required for its inhibitory activity against Proteus mirabilis C889 class A beta-lactamase | Citation and Details Article DOI: 10.1016/S0960-894X(01)80506-0 BindingDB Entry DOI: 10.7270/Q21V5HPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50469667 (6-Decylsalicylic Acid | CHEMBL416038) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article | n/a | n/a | 6.46E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Concentration required for its inhibitory activity against Proteus mirabilis C889 class A beta-lactamase | Citation and Details Article DOI: 10.1016/S0960-894X(01)80506-0 BindingDB Entry DOI: 10.7270/Q21V5HPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||