

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 1 [582-599] (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alexandria | Assay Description The compounds that exhibited potent anti-inflammatory profiles were futher tested for their ability to inhibit human COX-1 and COX-2 enzymes in-vitro... | J Enzyme Inhib Med Chem 24: 296-309 (2009) Article DOI: 10.1080/14756360802188404 BindingDB Entry DOI: 10.7270/Q2S1813W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 [582-599] (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 2.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
Shahid Beheshti University | Assay Description The ability of the test compounds to inhibit ovine COX-1 and COX-2 was determined using chemiluminescent enzyme assays kit according to our previousl... | Med Chem Res 19: 782-793 (2009) BindingDB Entry DOI: 10.7270/Q2T72G2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 [582-599] (Homo sapiens (Human)) | BDBM93106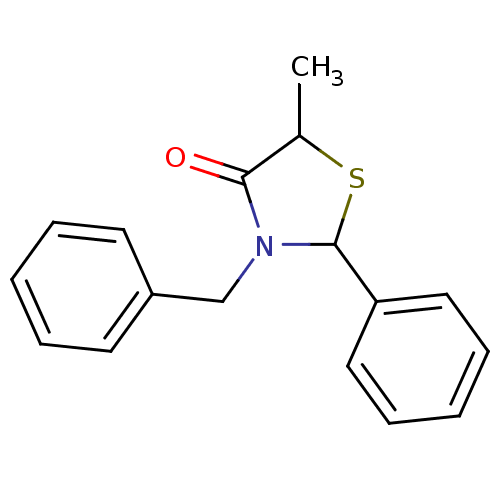 (COX inhibitor, 9) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | n/a | n/a | 7.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
Shahid Beheshti University | Assay Description The ability of the test compounds to inhibit ovine COX-1 and COX-2 was determined using chemiluminescent enzyme assays kit according to our previousl... | Med Chem Res 19: 782-793 (2009) BindingDB Entry DOI: 10.7270/Q2T72G2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 [582-599] (Homo sapiens (Human)) | BDBM91679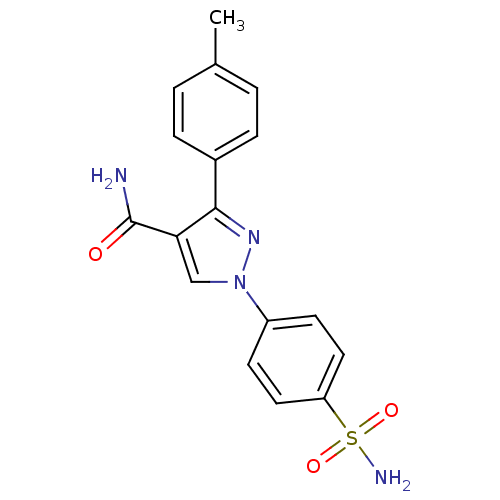 (Pyrazolyl benzenesulfonamide derivative, 7b) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alexandria | Assay Description The compounds that exhibited potent anti-inflammatory profiles were futher tested for their ability to inhibit human COX-1 and COX-2 enzymes in-vitro... | J Enzyme Inhib Med Chem 24: 296-309 (2009) Article DOI: 10.1080/14756360802188404 BindingDB Entry DOI: 10.7270/Q2S1813W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 [582-599] (Homo sapiens (Human)) | BDBM91678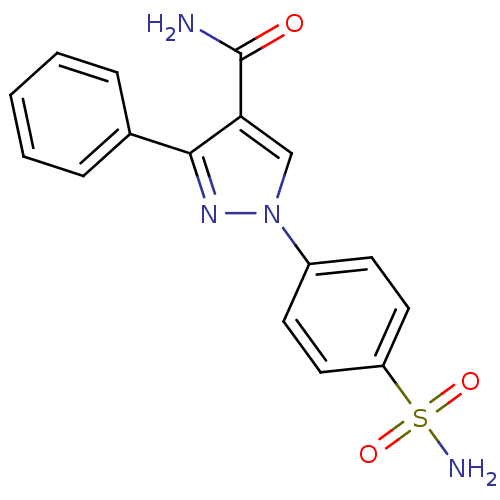 (Pyrazolyl benzenesulfonamide derivative, 7a) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alexandria | Assay Description The compounds that exhibited potent anti-inflammatory profiles were futher tested for their ability to inhibit human COX-1 and COX-2 enzymes in-vitro... | J Enzyme Inhib Med Chem 24: 296-309 (2009) Article DOI: 10.1080/14756360802188404 BindingDB Entry DOI: 10.7270/Q2S1813W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 [582-599] (Homo sapiens (Human)) | BDBM93105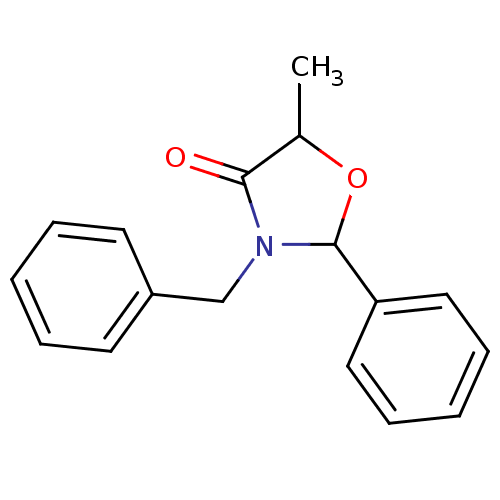 (COX inhibitor, 8) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 8.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
Shahid Beheshti University | Assay Description The ability of the test compounds to inhibit ovine COX-1 and COX-2 was determined using chemiluminescent enzyme assays kit according to our previousl... | Med Chem Res 19: 782-793 (2009) BindingDB Entry DOI: 10.7270/Q2T72G2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 [582-599] (Homo sapiens (Human)) | BDBM91685 (Pyrazolyl benzenesulfonamide derivative, 9b) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alexandria | Assay Description The compounds that exhibited potent anti-inflammatory profiles were futher tested for their ability to inhibit human COX-1 and COX-2 enzymes in-vitro... | J Enzyme Inhib Med Chem 24: 296-309 (2009) Article DOI: 10.1080/14756360802188404 BindingDB Entry DOI: 10.7270/Q2S1813W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 [582-599] (Homo sapiens (Human)) | BDBM93103 (COX inhibitor, 5a) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | |
Shahid Beheshti University | Assay Description The ability of the test compounds to inhibit ovine COX-1 and COX-2 was determined using chemiluminescent enzyme assays kit according to our previousl... | Med Chem Res 19: 782-793 (2009) BindingDB Entry DOI: 10.7270/Q2T72G2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 [582-599] (Homo sapiens (Human)) | BDBM93104 (COX inhibitor, 5b) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | |
Shahid Beheshti University | Assay Description The ability of the test compounds to inhibit ovine COX-1 and COX-2 was determined using chemiluminescent enzyme assays kit according to our previousl... | Med Chem Res 19: 782-793 (2009) BindingDB Entry DOI: 10.7270/Q2T72G2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 [582-599] (Homo sapiens (Human)) | BDBM91684 (Pyrazolyl benzenesulfonamide derivative, 9a) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alexandria | Assay Description The compounds that exhibited potent anti-inflammatory profiles were futher tested for their ability to inhibit human COX-1 and COX-2 enzymes in-vitro... | J Enzyme Inhib Med Chem 24: 296-309 (2009) Article DOI: 10.1080/14756360802188404 BindingDB Entry DOI: 10.7270/Q2S1813W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 [582-599] (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alexandria | Assay Description The compounds that exhibited potent anti-inflammatory profiles were futher tested for their ability to inhibit human COX-1 and COX-2 enzymes in-vitro... | J Enzyme Inhib Med Chem 24: 296-309 (2009) Article DOI: 10.1080/14756360802188404 BindingDB Entry DOI: 10.7270/Q2S1813W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||