 Found 15 hits of ic50 data for polymerid = 5404
Found 15 hits of ic50 data for polymerid = 5404 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase PAK 5
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PAK5 using RRRLSFAEPG as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.02.022
BindingDB Entry DOI: 10.7270/Q2DJ5KB8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 5
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human PAK5 using RRRLSFAEPG as substrate by [gamma-33P]-ATP assay |
Eur J Med Chem 161: 456-467 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.052
BindingDB Entry DOI: 10.7270/Q2W380MT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 5
(Homo sapiens (Human)) | BDBM50251098

(CHEMBL4097816)Show SMILES C[C@@H]1CN(CCN1)C(=O)c1nc(Nc2cc([nH]n2)C2CC2)c2cc(Cl)ccc2n1 |r| Show InChI InChI=1S/C20H22ClN7O/c1-11-10-28(7-6-22-11)20(29)19-23-15-5-4-13(21)8-14(15)18(25-19)24-17-9-16(26-27-17)12-2-3-12/h4-5,8-9,11-12,22H,2-3,6-7,10H2,1H3,(H2,23,24,25,26,27)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged PAK5 (295 to 591 residues) expressed in Baculovirus expression system by Z'-Lyte assay |
J Med Chem 61: 265-285 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01342
BindingDB Entry DOI: 10.7270/Q2BK1FSN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PAK 5
(Homo sapiens (Human)) | BDBM101618
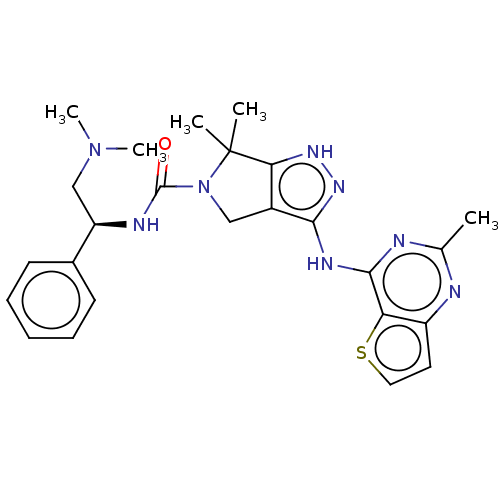
(US8530652, 114)Show SMILES CN(C)C[C@@H](NC(=O)N1Cc2c(Nc3nc(C)nc4ccsc34)n[nH]c2C1(C)C)c1ccccc1 Show InChI InChI=1S/C25H30N8OS/c1-15-26-18-11-12-35-20(18)23(27-15)29-22-17-13-33(25(2,3)21(17)30-31-22)24(34)28-19(14-32(4)5)16-9-7-6-8-10-16/h6-12,19H,13-14H2,1-5H3,(H,28,34)(H2,26,27,29,30,31)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PAK5 (unknown origin) using peptide 7 as substrate by pyruvate kinase/lactate dehydrogenase coupled assay |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PAK 5
(Homo sapiens (Human)) | BDBM50429615
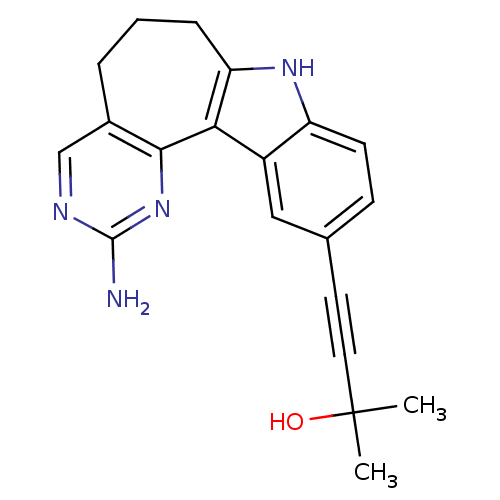
(CHEMBL2334586)Show SMILES CC(C)(O)C#Cc1ccc2[nH]c3CCCc4cnc(N)nc4-c3c2c1 Show InChI InChI=1S/C20H20N4O/c1-20(2,25)9-8-12-6-7-15-14(10-12)17-16(23-15)5-3-4-13-11-22-19(21)24-18(13)17/h6-7,10-11,23,25H,3-5H2,1-2H3,(H2,21,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00697
BindingDB Entry DOI: 10.7270/Q2MP5771 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 5
(Homo sapiens (Human)) | BDBM50448771
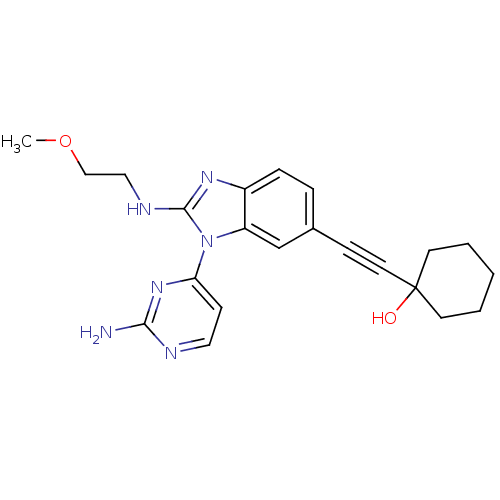
(CHEMBL3128042)Show SMILES COCCNc1nc2ccc(cc2n1-c1ccnc(N)n1)C#CC1(O)CCCCC1 Show InChI InChI=1S/C22H26N6O2/c1-30-14-13-25-21-26-17-6-5-16(7-11-22(29)9-3-2-4-10-22)15-18(17)28(21)19-8-12-24-20(23)27-19/h5-6,8,12,15,29H,2-4,9-10,13-14H2,1H3,(H,25,26)(H2,23,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK5 (unknown origin) |
J Med Chem 57: 1033-45 (2014)
Article DOI: 10.1021/jm401768t
BindingDB Entry DOI: 10.7270/Q2F47QNW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PAK 5
(Homo sapiens (Human)) | BDBM50400047
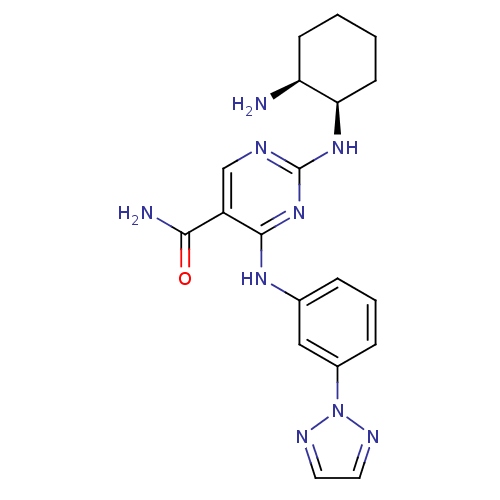
(BIIB-057 | CHEMBL2177736 | US9579320, Example 87)Show SMILES N[C@H]1CCCC[C@H]1Nc1ncc(C(N)=O)c(Nc2cccc(c2)-n2nccn2)n1 |r| Show InChI InChI=1S/C19H23N9O/c20-15-6-1-2-7-16(15)26-19-22-11-14(17(21)29)18(27-19)25-12-4-3-5-13(10-12)28-23-8-9-24-28/h3-5,8-11,15-16H,1-2,6-7,20H2,(H2,21,29)(H2,22,25,26,27)/t15-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a |
Portola Pharmaceuticals, Inc.
US Patent
| Assay Description
Employing the Milipore panel of purified kinases EXAMPLE 87 (IC50=1 nM) inhibited 98% of purified Syk kinase activity at 50 nM. IC50 values were dete... |
US Patent US8952027 (2015)
BindingDB Entry DOI: 10.7270/Q2CF9NV0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 5
(Homo sapiens (Human)) | BDBM150751
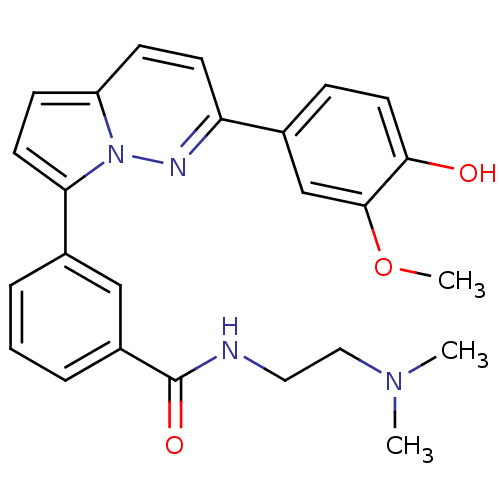
(CPR005231 (6))Show SMILES COc1cc(ccc1O)-c1ccc2ccc(-c3cccc(c3)C(=O)NCCN(C)C)n2n1 Show InChI InChI=1S/C25H26N4O3/c1-28(2)14-13-26-25(31)19-6-4-5-18(15-19)22-11-9-20-8-10-21(27-29(20)22)17-7-12-23(30)24(16-17)32-3/h4-12,15-16,30H,13-14H2,1-3H3,(H,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Copenhagen
| Assay Description
The reaction was started by adding a mix of ATP and Peptide 38 to assay plates containing DAPK1. Prior to mixing, all reagents are diluted in assay b... |
Chembiochem 16: 59-63 (2015)
Article DOI: 10.1002/cbic.201402512
BindingDB Entry DOI: 10.7270/Q2XW4HHD |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 5
(Homo sapiens (Human)) | BDBM50135286

(CHEMBL3745885)Show SMILES Cn1c2nc(Nc3ccc4[nH]ccc4c3)ncc2cc(c1=O)S(=O)(=O)c1ccc(F)cc1F Show InChI InChI=1S/C22H15F2N5O3S/c1-29-20-13(9-19(21(29)30)33(31,32)18-5-2-14(23)10-16(18)24)11-26-22(28-20)27-15-3-4-17-12(8-15)6-7-25-17/h2-11,25H,1H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
| Assay Description
Inhibition of human PAK5 using [KKLNRTLSFAEPG] substrate |
Bioorg Med Chem 24: 521-44 (2016)
Article DOI: 10.1016/j.bmc.2015.11.045
BindingDB Entry DOI: 10.7270/Q24Q7WT8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 5
(Homo sapiens (Human)) | BDBM50112354
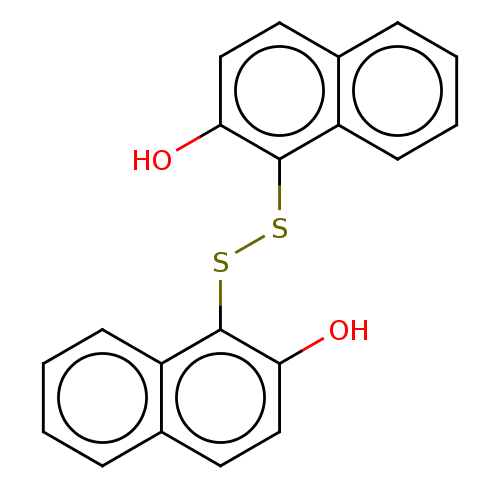
(CHEMBL472940)Show InChI InChI=1S/C20H14O2S2/c21-17-11-9-13-5-1-3-7-15(13)19(17)23-24-20-16-8-4-2-6-14(16)10-12-18(20)22/h1-12,21-22H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length PAK5 (unknown origin) expressed in HEK293 cells assessed as phosphate incorporation onto MBP preincubated for 5 mins follow... |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 5
(Homo sapiens (Human)) | BDBM50519662
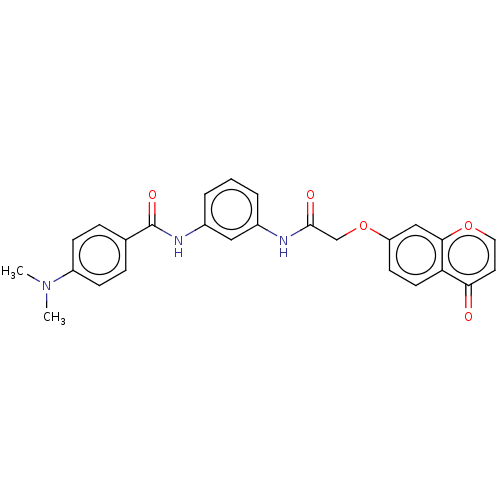
(CHEMBL4438748)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1cccc(NC(=O)COc2ccc3c(c2)occc3=O)c1 Show InChI InChI=1S/C26H23N3O5/c1-29(2)20-8-6-17(7-9-20)26(32)28-19-5-3-4-18(14-19)27-25(31)16-34-21-10-11-22-23(30)12-13-33-24(22)15-21/h3-15H,16H2,1-2H3,(H,27,31)(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PAK5 (425 to end residues) using RRRLSFAEPG as substrate measured after 40 mins in presence of [gamma33P]ATP by radio... |
J Med Chem 62: 10691-10710 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01143
BindingDB Entry DOI: 10.7270/Q2MC93FG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 5
(Homo sapiens (Human)) | BDBM93207
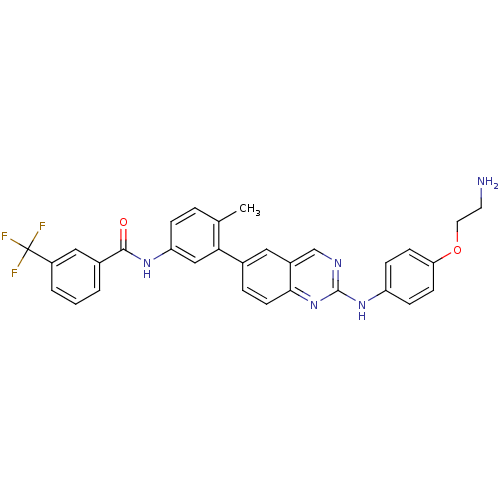
(Kinase inhibitor, 5)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1-c1ccc2nc(Nc3ccc(OCCN)cc3)ncc2c1 Show InChI InChI=1S/C31H26F3N5O2/c1-19-5-7-25(37-29(40)21-3-2-4-23(16-21)31(32,33)34)17-27(19)20-6-12-28-22(15-20)18-36-30(39-28)38-24-8-10-26(11-9-24)41-14-13-35/h2-12,15-18H,13-14,35H2,1H3,(H,37,40)(H,36,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Washington
| Assay Description
Fluorescence assay used for determination of catch and release efficiency. |
ACS Chem Biol 8: 691-9 (2013)
Article DOI: 10.1021/cb300623a
BindingDB Entry DOI: 10.7270/Q20R9N1X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 5
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of PAK5 |
Bioorg Med Chem Lett 21: 7155-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.078
BindingDB Entry DOI: 10.7270/Q2NC61NH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 5
(Homo sapiens (Human)) | BDBM50359359

(CHEMBL1929238)Show SMILES CN(C)CCN1CCN(CCC1=O)C(=O)c1cc(sc1NC(=O)Nc1cccc(Cl)c1Cl)C(C)(C)C Show InChI InChI=1S/C25H33Cl2N5O3S/c1-25(2,3)19-15-16(22(36-19)29-24(35)28-18-8-6-7-17(26)21(18)27)23(34)32-10-9-20(33)31(13-14-32)12-11-30(4)5/h6-8,15H,9-14H2,1-5H3,(H2,28,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of PAK5 |
Bioorg Med Chem Lett 21: 7155-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.078
BindingDB Entry DOI: 10.7270/Q2NC61NH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 5
(Homo sapiens (Human)) | BDBM50537742
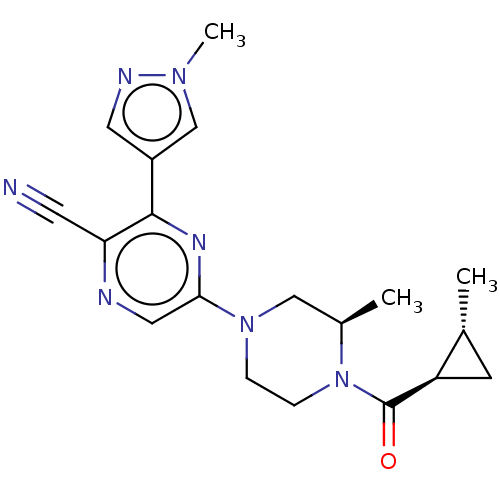
(CHEMBL4634634 | US11179389, Compound 1-14)Show SMILES C[C@@H]1C[C@H]1C(=O)N1CCN(C[C@H]1C)c1cnc(C#N)c(n1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C19H23N7O/c1-12-6-15(12)19(27)26-5-4-25(10-13(26)2)17-9-21-16(7-20)18(23-17)14-8-22-24(3)11-14/h8-9,11-13,15H,4-6,10H2,1-3H3/t12-,13-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged PAK7 catalytic domain (425 to 719 residues) expressed in baculovirus expression system using serine/threon... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126715
BindingDB Entry DOI: 10.7270/Q2HM5CZG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data