 Found 705 hits of ki for UniProtKB: P25774
Found 705 hits of ki for UniProtKB: P25774 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin S
(Homo sapiens (Human)) | BDBM50546794

(CHEMBL4777335)Show SMILES CC(C)(C)CC(=O)N[C@@H](CS(=O)(=O)N1CCN(CC1)c1ccccn1)C(=O)NC1(CC1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01803
BindingDB Entry DOI: 10.7270/Q2KD22XQ |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50546794

(CHEMBL4777335)Show SMILES CC(C)(C)CC(=O)N[C@@H](CS(=O)(=O)N1CCN(CC1)c1ccccn1)C(=O)NC1(CC1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00949
BindingDB Entry DOI: 10.7270/Q2NV9NVM |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50243232

(CHEMBL486232 | GNF-PF-5434 | N-((S)-4-methyl-1-oxo...)Show SMILES CC(C)C[C@H](NC(=O)N1CCOCC1)C(=O)N[C@@H](CCc1ccccc1)C=CS(=O)(=O)c1ccccc1 |r,w:27.29| Show InChI InChI=1S/C28H37N3O5S/c1-22(2)21-26(30-28(33)31-16-18-36-19-17-31)27(32)29-24(14-13-23-9-5-3-6-10-23)15-20-37(34,35)25-11-7-4-8-12-25/h3-12,15,20,22,24,26H,13-14,16-19,21H2,1-2H3,(H,29,32)(H,30,33)/t24-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S by fluorescence assay |
Bioorg Med Chem 17: 1064-70 (2009)
Article DOI: 10.1016/j.bmc.2008.02.002
BindingDB Entry DOI: 10.7270/Q20R9P6K |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50030745
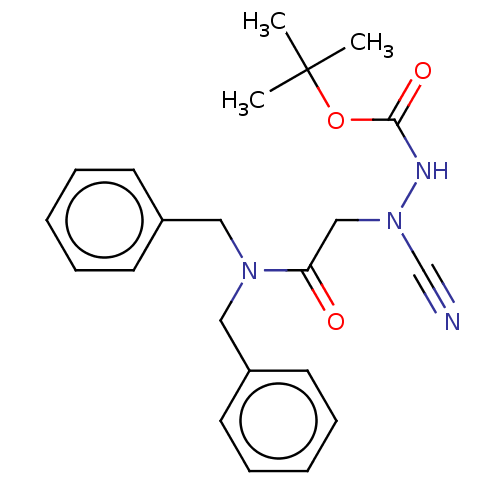
(CHEMBL3342185 | acs.jmedchem.1c00409_ST.412)Show SMILES CC(C)(C)OC(=O)NN(CC(=O)N(Cc1ccccc1)Cc1ccccc1)C#N Show InChI InChI=1S/C22H26N4O3/c1-22(2,3)29-21(28)24-26(17-23)16-20(27)25(14-18-10-6-4-7-11-18)15-19-12-8-5-9-13-19/h4-13H,14-16H2,1-3H3,(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S using Z-Phe-Arg-AMC fluorogenic substrate fluorogenic substrate incubated for 60 mins |
ACS Med Chem Lett 5: 1076-81 (2014)
Article DOI: 10.1021/ml500238q
BindingDB Entry DOI: 10.7270/Q20P11NT |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50335289
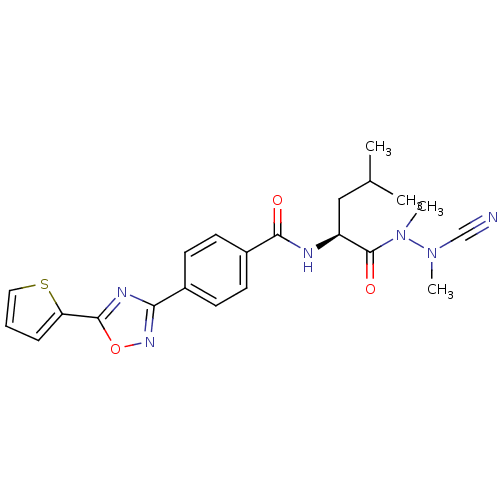
(CHEMBL1651361 | N-{4-[5-(2-Thienyl)-1,2,4-oxadiazo...)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)-c1noc(n1)-c1cccs1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C22H24N6O3S/c1-14(2)12-17(22(30)28(4)27(3)13-23)24-20(29)16-9-7-15(8-10-16)19-25-21(31-26-19)18-6-5-11-32-18/h5-11,14,17H,12H2,1-4H3,(H,24,29)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50335281

(CHEMBL1651355 | N-(Phenylcarbamoyl)-leucyl-methyla...)Show SMILES CC(C)C[C@H](NC(=O)Nc1ccccc1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C16H23N5O2/c1-12(2)10-14(15(22)21(4)20(3)11-17)19-16(23)18-13-8-6-5-7-9-13/h5-9,12,14H,10H2,1-4H3,(H2,18,19,23)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50335283

(CHEMBL1651357 | N-{4-[5-(2-Thienyl)-1,2,4-oxadiazo...)Show SMILES CC(C)C[C@H](NC(=O)NCc1ccc(cc1)-c1noc(n1)-c1cccs1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C23H27N7O3S/c1-15(2)12-18(22(31)30(4)29(3)14-24)26-23(32)25-13-16-7-9-17(10-8-16)20-27-21(33-28-20)19-6-5-11-34-19/h5-11,15,18H,12-13H2,1-4H3,(H2,25,26,32)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50335282
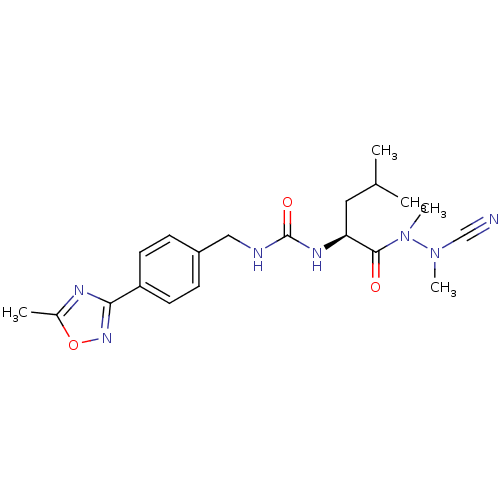
(CHEMBL1651356 | N-[4-(5-Methyl-1,2,4-oxadiazol-3-y...)Show SMILES CC(C)C[C@H](NC(=O)NCc1ccc(cc1)-c1noc(C)n1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C20H27N7O3/c1-13(2)10-17(19(28)27(5)26(4)12-21)24-20(29)22-11-15-6-8-16(9-7-15)18-23-14(3)30-25-18/h6-9,13,17H,10-11H2,1-5H3,(H2,22,24,29)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50030746
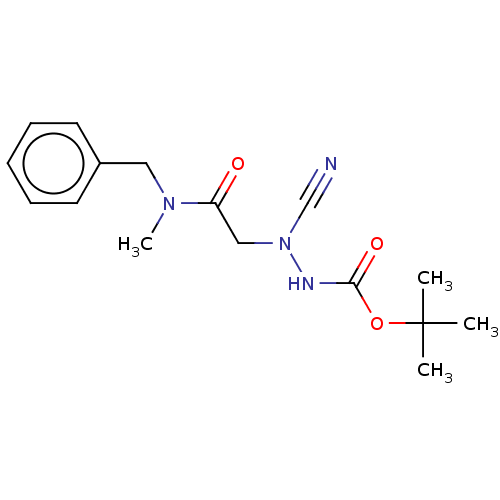
(CHEMBL3342184 | acs.jmedchem.1c00409_ST.413)Show InChI InChI=1S/C16H22N4O3/c1-16(2,3)23-15(22)18-20(12-17)11-14(21)19(4)10-13-8-6-5-7-9-13/h5-9H,10-11H2,1-4H3,(H,18,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.176 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S using Z-Phe-Arg-AMC fluorogenic substrate fluorogenic substrate incubated for 60 mins |
ACS Med Chem Lett 5: 1076-81 (2014)
Article DOI: 10.1021/ml500238q
BindingDB Entry DOI: 10.7270/Q20P11NT |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50335284
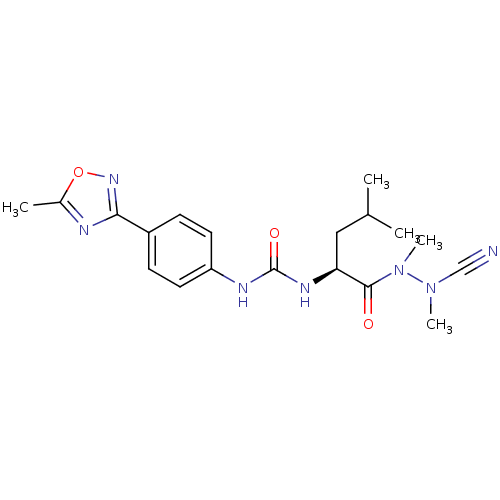
(CHEMBL1651349 | N-[4-(5-Methyl-1,2,4-oxadiazol-3-y...)Show SMILES CC(C)C[C@H](NC(=O)Nc1ccc(cc1)-c1noc(C)n1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C19H25N7O3/c1-12(2)10-16(18(27)26(5)25(4)11-20)23-19(28)22-15-8-6-14(7-9-15)17-21-13(3)29-24-17/h6-9,12,16H,10H2,1-5H3,(H2,22,23,28)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50335278

(CHEMBL1651352 | N-(Benzyloxycarbonyl)-cyclohexylal...)Show SMILES CN(C#N)N(C)C(=O)[C@H](CC1CCCCC1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C20H28N4O3/c1-23(15-21)24(2)19(25)18(13-16-9-5-3-6-10-16)22-20(26)27-14-17-11-7-4-8-12-17/h4,7-8,11-12,16,18H,3,5-6,9-10,13-14H2,1-2H3,(H,22,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50546795
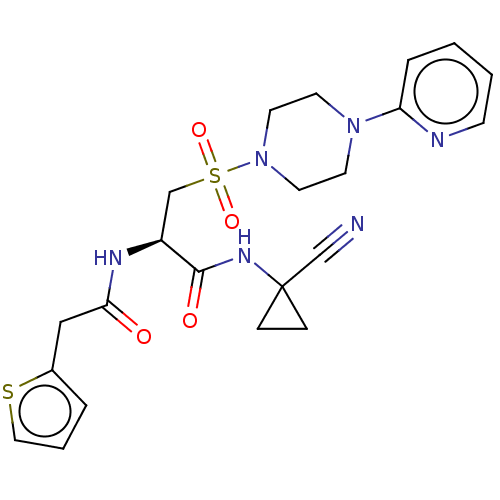
(CHEMBL4777740)Show SMILES O=C(Cc1cccs1)N[C@@H](CS(=O)(=O)N1CCN(CC1)c1ccccn1)C(=O)NC1(CC1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00949
BindingDB Entry DOI: 10.7270/Q2NV9NVM |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50335280

(CHEMBL1651354 | N-(Benzylcarbamoyl)-leucyl-methyla...)Show SMILES CC(C)C[C@H](NC(=O)NCc1ccccc1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C17H25N5O2/c1-13(2)10-15(16(23)22(4)21(3)12-18)20-17(24)19-11-14-8-6-5-7-9-14/h5-9,13,15H,10-11H2,1-4H3,(H2,19,20,24)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50546796
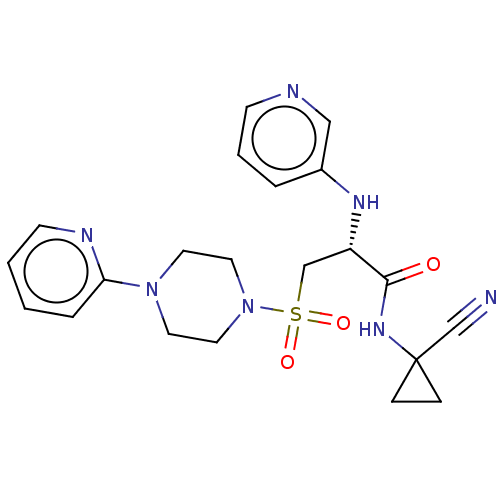
(CHEMBL4761229)Show SMILES O=C(NC1(CC1)C#N)[C@H](CS(=O)(=O)N1CCN(CC1)c1ccccn1)Nc1cccnc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00949
BindingDB Entry DOI: 10.7270/Q2NV9NVM |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50410979
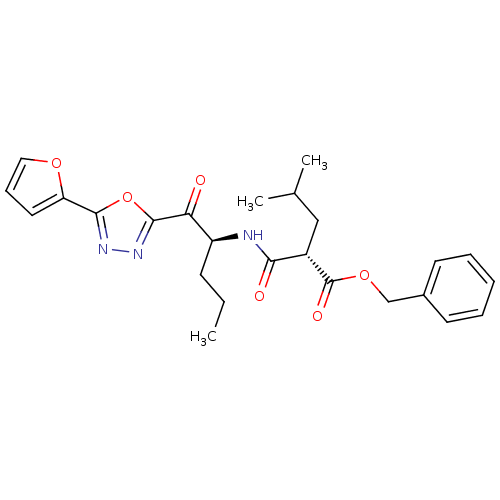
(CHEMBL207347)Show SMILES CCC[C@H](NC(=O)[C@H](CC(C)C)C(=O)OCc1ccccc1)C(=O)c1nnc(o1)-c1ccco1 Show InChI InChI=1S/C25H29N3O6/c1-4-9-19(21(29)24-28-27-23(34-24)20-12-8-13-32-20)26-22(30)18(14-16(2)3)25(31)33-15-17-10-6-5-7-11-17/h5-8,10-13,16,18-19H,4,9,14-15H2,1-3H3,(H,26,30)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 16: 2909-14 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.001
BindingDB Entry DOI: 10.7270/Q2QN6808 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50084650
(CHEMBL177914 | {(S)-1-[(S)-1-Formyl-2-(4-hydroxy-p...)Show SMILES Oc1ccc(C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)OCc2ccccc2)C=O)cc1 Show InChI InChI=1S/C26H26N2O5/c29-17-22(15-20-11-13-23(30)14-12-20)27-25(31)24(16-19-7-3-1-4-8-19)28-26(32)33-18-21-9-5-2-6-10-21/h1-14,17,22,24,30H,15-16,18H2,(H,27,31)(H,28,32)/t22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin S |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM109847

(US8609681, 70)Show SMILES FC(F)(C[C@H](NC(=O)N1CCC2(CC2)CC1)C(=O)NC1(CC1)C#N)Cc1ccccc1 |r| Show InChI InChI=1S/C23H28F2N4O2/c24-23(25,14-17-4-2-1-3-5-17)15-18(19(30)28-22(16-26)8-9-22)27-20(31)29-12-10-21(6-7-21)11-13-29/h1-5,18H,6-15H2,(H,27,31)(H,28,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Determination of the enzymatic activity of the catalytic domain of human Cathepsin S. This protein is obtained as an inactive enzyme from R&D Systems... |
US Patent US8609681 (2013)
BindingDB Entry DOI: 10.7270/Q2F47MSF |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50335285
(CHEMBL1651350 | N-{4-[5-(2-Thienyl)-1,2,4-oxadiazo...)Show SMILES CC(C)C[C@H](NC(=O)Nc1ccc(cc1)-c1noc(n1)-c1cccs1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C22H25N7O3S/c1-14(2)12-17(21(30)29(4)28(3)13-23)25-22(31)24-16-9-7-15(8-10-16)19-26-20(32-27-19)18-6-5-11-33-18/h5-11,14,17H,12H2,1-4H3,(H2,24,25,31)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50304794

((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-4-me...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C17H24N4O3/c1-13(2)10-15(16(22)21(4)20(3)12-18)19-17(23)24-11-14-8-6-5-7-9-14/h5-9,13,15H,10-11H2,1-4H3,(H,19,23)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM103367
(US8552202, Example 4)Show SMILES C[C@H]1CC[C@@H](CC1)[C@H](NC(=O)c1cccc(c1)-n1ccnc1)C(=O)N1C[C@H](Cl)[C@H]2OCC(=O)[C@@H]12 |r,wU:7.8,33.36,28.31,4.4,wD:26.29,1.0,(1.93,4.62,;1.93,3.08,;.6,2.31,;.6,.77,;1.93,,;3.27,.77,;3.27,2.31,;1.93,-1.54,;.6,-2.31,;-.73,-1.54,;-.73,,;-2.07,-2.31,;-2.07,-3.85,;-3.4,-4.62,;-4.73,-3.85,;-4.73,-2.31,;-3.4,-1.54,;-6.07,-1.54,;-7.53,-2.02,;-8.44,-.77,;-7.53,.48,;-6.07,,;3.27,-2.31,;3.27,-3.85,;4.6,-1.54,;4.6,,;6.07,.48,;6.84,1.81,;6.97,-.77,;8.44,-1.25,;8.44,-2.79,;6.97,-3.26,;6.2,-4.6,;6.07,-2.02,)| Show InChI InChI=1S/C25H29ClN4O4/c1-15-5-7-16(8-6-15)21(25(33)30-12-19(26)23-22(30)20(31)13-34-23)28-24(32)17-3-2-4-18(11-17)29-10-9-27-14-29/h2-4,9-11,14-16,19,21-23H,5-8,12-13H2,1H3,(H,28,32)/t15-,16-,19-,21-,22+,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM109859
(US8609681, 102)Show SMILES CCCC(F)(F)C[C@H](NC(=O)N1CCC2(CC2)CC1)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C19H28F2N4O2/c1-2-3-19(20,21)12-14(15(26)24-18(13-22)6-7-18)23-16(27)25-10-8-17(4-5-17)9-11-25/h14H,2-12H2,1H3,(H,23,27)(H,24,26)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Determination of the enzymatic activity of the catalytic domain of human Cathepsin S. This protein is obtained as an inactive enzyme from R&D Systems... |
US Patent US8609681 (2013)
BindingDB Entry DOI: 10.7270/Q2F47MSF |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50304793
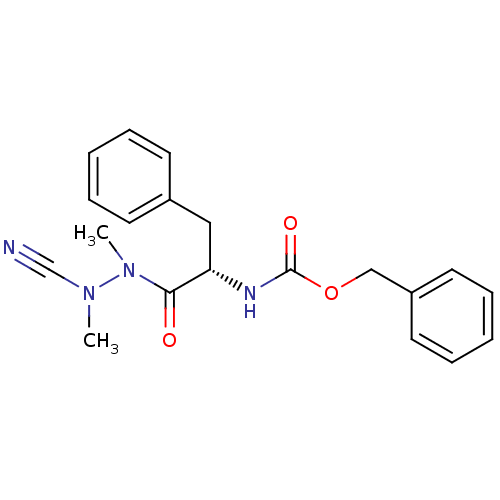
((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-1-ox...)Show SMILES CN(C#N)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C20H22N4O3/c1-23(15-21)24(2)19(25)18(13-16-9-5-3-6-10-16)22-20(26)27-14-17-11-7-4-8-12-17/h3-12,18H,13-14H2,1-2H3,(H,22,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50392215
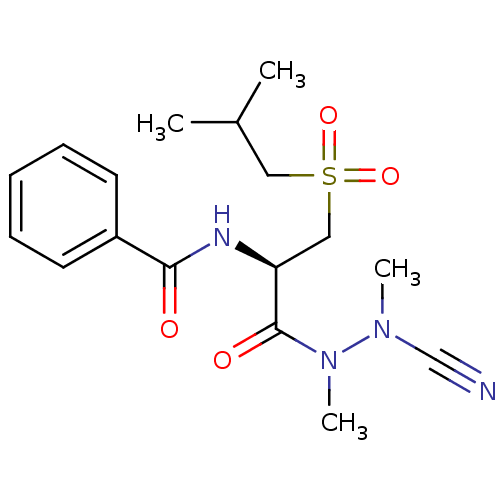
(CHEMBL2153161)Show SMILES CC(C)CS(=O)(=O)C[C@H](NC(=O)c1ccccc1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C17H24N4O4S/c1-13(2)10-26(24,25)11-15(17(23)21(4)20(3)12-18)19-16(22)14-8-6-5-7-9-14/h5-9,13,15H,10-11H2,1-4H3,(H,19,22)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S using Z-Phe-Val-Arg-pNA as substrate after 80 mins by spectrophotometric analysis |
J Med Chem 55: 5982-6 (2012)
Article DOI: 10.1021/jm300734k
BindingDB Entry DOI: 10.7270/Q2833T40 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50002399
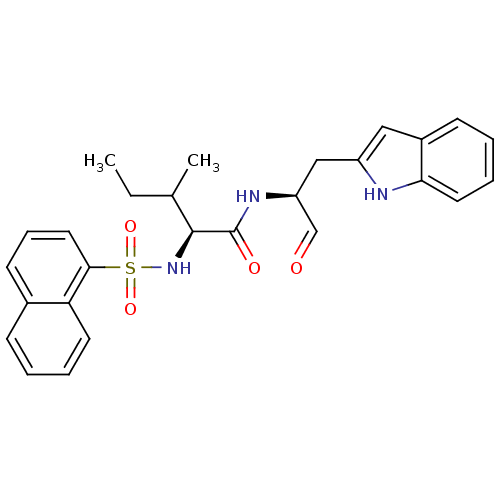
(CHEMBL197958)Show SMILES CCC(C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@@H](Cc1cc2ccccc2[nH]1)C=O Show InChI InChI=1S/C27H29N3O4S/c1-3-18(2)26(30-35(33,34)25-14-8-11-19-9-4-6-12-23(19)25)27(32)29-22(17-31)16-21-15-20-10-5-7-13-24(20)28-21/h4-15,17-18,22,26,28,30H,3,16H2,1-2H3,(H,29,32)/t18?,22-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin S |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50255571
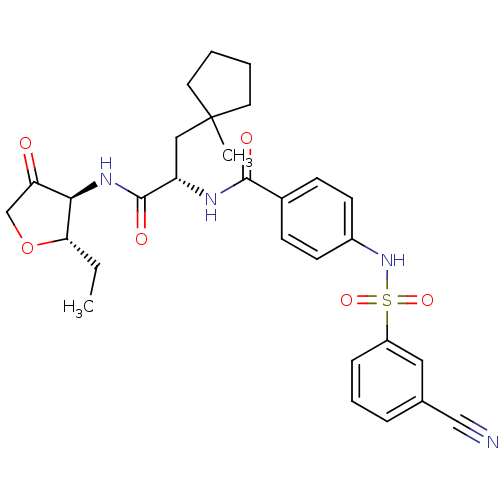
(4-(3-Cyano-benzenesulfonylamino)-N-[1-(2S-ethyl-4-...)Show SMILES CC[C@@H]1OCC(=O)[C@H]1NC(=O)[C@H](CC1(C)CCCC1)NC(=O)c1ccc(NS(=O)(=O)c2cccc(c2)C#N)cc1 |r| Show InChI InChI=1S/C29H34N4O6S/c1-3-25-26(24(34)18-39-25)32-28(36)23(16-29(2)13-4-5-14-29)31-27(35)20-9-11-21(12-10-20)33-40(37,38)22-8-6-7-19(15-22)17-30/h6-12,15,23,25-26,33H,3-5,13-14,16,18H2,1-2H3,(H,31,35)(H,32,36)/t23-,25-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S expressed in baculovirus by fluorescence assay |
Bioorg Med Chem 17: 1307-24 (2009)
Article DOI: 10.1016/j.bmc.2008.12.020
BindingDB Entry DOI: 10.7270/Q2GX4BD9 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50255790
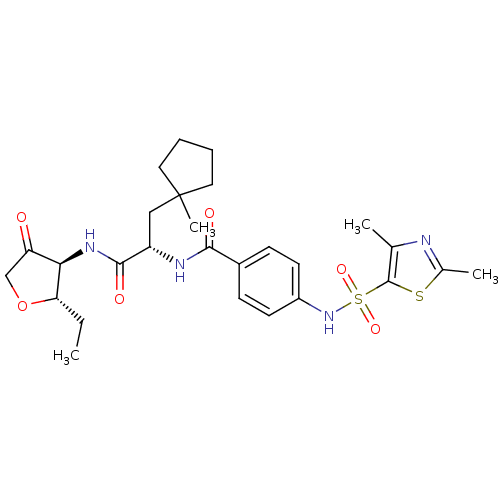
(4-(2,4-Dimethyl-thiazole-5-sulfonylamino)-N-[1-(2S...)Show SMILES CC[C@@H]1OCC(=O)[C@H]1NC(=O)[C@H](CC1(C)CCCC1)NC(=O)c1ccc(NS(=O)(=O)c2sc(C)nc2C)cc1 |r| Show InChI InChI=1S/C27H36N4O6S2/c1-5-22-23(21(32)15-37-22)30-25(34)20(14-27(4)12-6-7-13-27)29-24(33)18-8-10-19(11-9-18)31-39(35,36)26-16(2)28-17(3)38-26/h8-11,20,22-23,31H,5-7,12-15H2,1-4H3,(H,29,33)(H,30,34)/t20-,22-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S expressed in baculovirus by fluorescence assay |
Bioorg Med Chem 17: 1307-24 (2009)
Article DOI: 10.1016/j.bmc.2008.12.020
BindingDB Entry DOI: 10.7270/Q2GX4BD9 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50030747
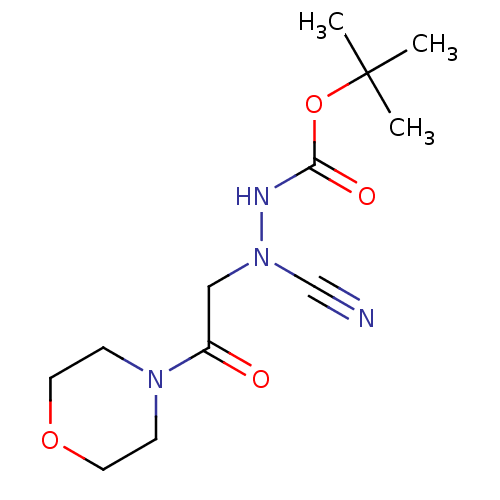
(CHEMBL3342183)Show InChI InChI=1S/C12H20N4O4/c1-12(2,3)20-11(18)14-16(9-13)8-10(17)15-4-6-19-7-5-15/h4-8H2,1-3H3,(H,14,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.777 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S using Z-Phe-Arg-AMC fluorogenic substrate fluorogenic substrate incubated for 60 mins |
ACS Med Chem Lett 5: 1076-81 (2014)
Article DOI: 10.1021/ml500238q
BindingDB Entry DOI: 10.7270/Q20P11NT |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50255627
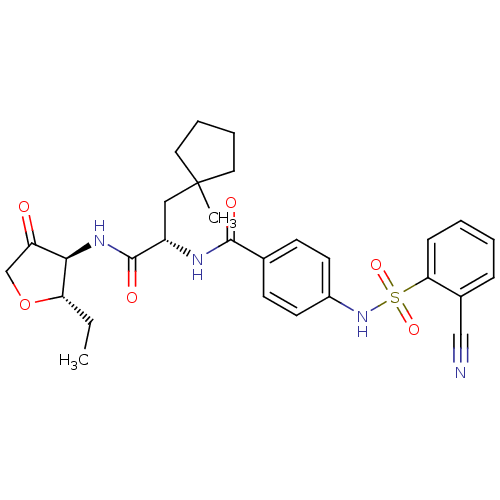
(4-(2-Cyano-benzenesulfonylamino)-N-[1-(2S-ethyl-4-...)Show SMILES CC[C@@H]1OCC(=O)[C@H]1NC(=O)[C@H](CC1(C)CCCC1)NC(=O)c1ccc(NS(=O)(=O)c2ccccc2C#N)cc1 |r| Show InChI InChI=1S/C29H34N4O6S/c1-3-24-26(23(34)18-39-24)32-28(36)22(16-29(2)14-6-7-15-29)31-27(35)19-10-12-21(13-11-19)33-40(37,38)25-9-5-4-8-20(25)17-30/h4-5,8-13,22,24,26,33H,3,6-7,14-16,18H2,1-2H3,(H,31,35)(H,32,36)/t22-,24-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S expressed in baculovirus by fluorescence assay |
Bioorg Med Chem 17: 1307-24 (2009)
Article DOI: 10.1016/j.bmc.2008.12.020
BindingDB Entry DOI: 10.7270/Q2GX4BD9 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50255133

(4-Benzenesulfonylamino-N-[1-(2S-ethyl-4-oxo-tetrah...)Show SMILES CC[C@@H]1OCC(=O)[C@H]1NC(=O)[C@H](CC1(C)CCCC1)NC(=O)c1ccc(NS(=O)(=O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C28H35N3O6S/c1-3-24-25(23(32)18-37-24)30-27(34)22(17-28(2)15-7-8-16-28)29-26(33)19-11-13-20(14-12-19)31-38(35,36)21-9-5-4-6-10-21/h4-6,9-14,22,24-25,31H,3,7-8,15-18H2,1-2H3,(H,29,33)(H,30,34)/t22-,24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S expressed in baculovirus by fluorescence assay |
Bioorg Med Chem 17: 1307-24 (2009)
Article DOI: 10.1016/j.bmc.2008.12.020
BindingDB Entry DOI: 10.7270/Q2GX4BD9 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50335277

(CHEMBL1651351 | N-(Benzyloxycarbonyl)-isoleucyl-me...)Show SMILES CC[C@H](C)[C@H](NC(=O)OCc1ccccc1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C17H24N4O3/c1-5-13(2)15(16(22)21(4)20(3)12-18)19-17(23)24-11-14-9-7-6-8-10-14/h6-10,13,15H,5,11H2,1-4H3,(H,19,23)/t13-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50335279
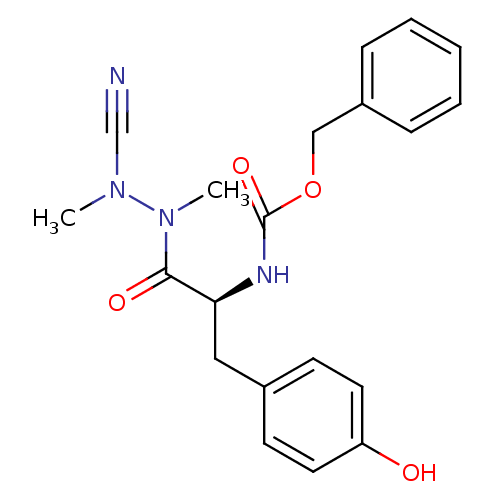
(CHEMBL1651353 | N-(Benzyloxycarbonyl)-tyrosyl-meth...)Show SMILES CN(C#N)N(C)C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C20H22N4O4/c1-23(14-21)24(2)19(26)18(12-15-8-10-17(25)11-9-15)22-20(27)28-13-16-6-4-3-5-7-16/h3-11,18,25H,12-13H2,1-2H3,(H,22,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
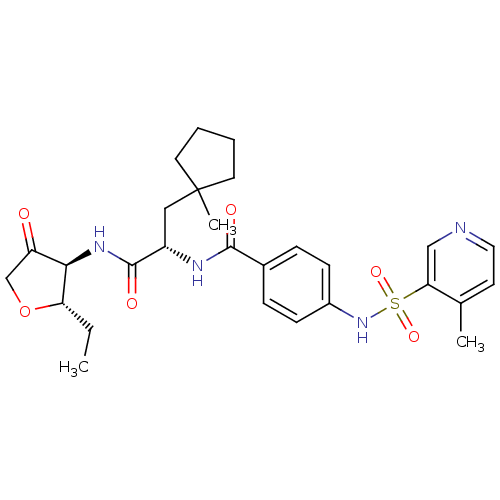
Cathepsin S
(Homo sapiens (Human)) | BDBM50255681
(CHEMBL473448 | N-[1-(2S-Ethyl-4-oxo-tetrahydro-fur...)Show SMILES CC[C@@H]1OCC(=O)[C@H]1NC(=O)[C@H](CC1(C)CCCC1)NC(=O)c1ccc(NS(=O)(=O)c2cnccc2C)cc1 |r| Show InChI InChI=1S/C28H36N4O6S/c1-4-23-25(22(33)17-38-23)31-27(35)21(15-28(3)12-5-6-13-28)30-26(34)19-7-9-20(10-8-19)32-39(36,37)24-16-29-14-11-18(24)2/h7-11,14,16,21,23,25,32H,4-6,12-13,15,17H2,1-3H3,(H,30,34)(H,31,35)/t21-,23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S expressed in baculovirus by fluorescence assay |
Bioorg Med Chem 17: 1307-24 (2009)
Article DOI: 10.1016/j.bmc.2008.12.020
BindingDB Entry DOI: 10.7270/Q2GX4BD9 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM109858
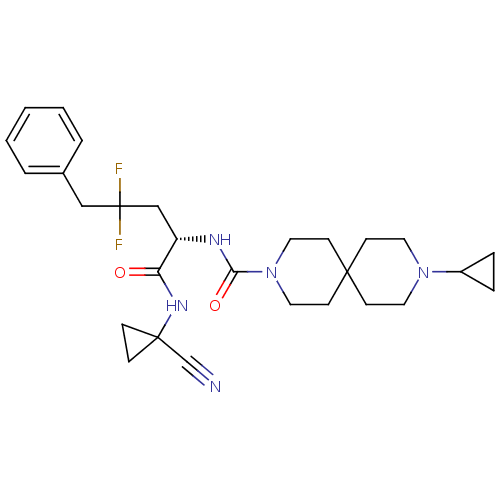
(US8609681, 97)Show SMILES FC(F)(C[C@H](NC(=O)N1CCC2(CCN(CC2)C2CC2)CC1)C(=O)NC1(CC1)C#N)Cc1ccccc1 |r| Show InChI InChI=1S/C28H37F2N5O2/c29-28(30,18-21-4-2-1-3-5-21)19-23(24(36)33-27(20-31)8-9-27)32-25(37)35-16-12-26(13-17-35)10-14-34(15-11-26)22-6-7-22/h1-5,22-23H,6-19H2,(H,32,37)(H,33,36)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Determination of the enzymatic activity of the catalytic domain of human Cathepsin S. This protein is obtained as an inactive enzyme from R&D Systems... |
US Patent US8609681 (2013)
BindingDB Entry DOI: 10.7270/Q2F47MSF |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19622
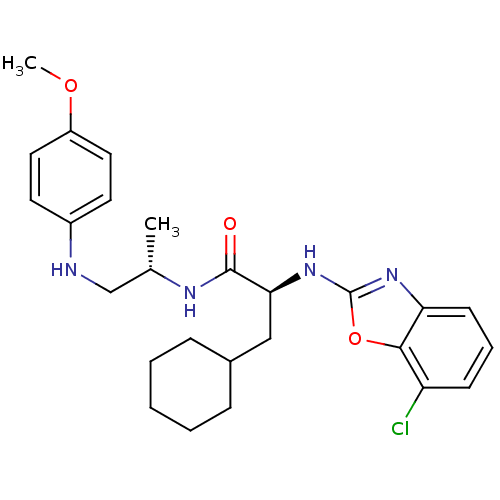
((2S)-2-[(7-chloro-1,3-benzoxazol-2-yl)amino]-3-cyc...)Show SMILES COc1ccc(NC[C@H](C)NC(=O)[C@H](CC2CCCCC2)Nc2nc3cccc(Cl)c3o2)cc1 |r| Show InChI InChI=1S/C26H33ClN4O3/c1-17(16-28-19-11-13-20(33-2)14-12-19)29-25(32)23(15-18-7-4-3-5-8-18)31-26-30-22-10-6-9-21(27)24(22)34-26/h6,9-14,17-18,23,28H,3-5,7-8,15-16H2,1-2H3,(H,29,32)(H,30,31)/t17-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | -12.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 16: 1975-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.095
BindingDB Entry DOI: 10.7270/Q2PZ573M |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50255569

(CHEMBL475669 | N-[1-(2S-Ethyl-4-oxo-tetrahydro-fur...)Show SMILES CC[C@@H]1OCC(=O)[C@H]1NC(=O)[C@H](CC1(C)CCCC1)NC(=O)c1ccc(NS(=O)(=O)c2cccc(OC)c2)cc1 |r| Show InChI InChI=1S/C29H37N3O7S/c1-4-25-26(24(33)18-39-25)31-28(35)23(17-29(2)14-5-6-15-29)30-27(34)19-10-12-20(13-11-19)32-40(36,37)22-9-7-8-21(16-22)38-3/h7-13,16,23,25-26,32H,4-6,14-15,17-18H2,1-3H3,(H,30,34)(H,31,35)/t23-,25-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S expressed in baculovirus by fluorescence assay |
Bioorg Med Chem 17: 1307-24 (2009)
Article DOI: 10.1016/j.bmc.2008.12.020
BindingDB Entry DOI: 10.7270/Q2GX4BD9 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50255570

(4-(4-Cyano-benzenesulfonylamino)-N-[1-(2S-ethyl-4-...)Show SMILES CC[C@@H]1OCC(=O)[C@H]1NC(=O)[C@H](CC1(C)CCCC1)NC(=O)c1ccc(NS(=O)(=O)c2ccc(cc2)C#N)cc1 |r| Show InChI InChI=1S/C29H34N4O6S/c1-3-25-26(24(34)18-39-25)32-28(36)23(16-29(2)14-4-5-15-29)31-27(35)20-8-10-21(11-9-20)33-40(37,38)22-12-6-19(17-30)7-13-22/h6-13,23,25-26,33H,3-5,14-16,18H2,1-2H3,(H,31,35)(H,32,36)/t23-,25-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S expressed in baculovirus by fluorescence assay |
Bioorg Med Chem 17: 1307-24 (2009)
Article DOI: 10.1016/j.bmc.2008.12.020
BindingDB Entry DOI: 10.7270/Q2GX4BD9 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50255499

(4-(4-Amino-benzenesulfonylamino)-N-[1-(2S-ethyl-4-...)Show SMILES CC[C@@H]1OCC(=O)[C@H]1NC(=O)[C@H](CC1(C)CCCC1)NC(=O)c1ccc(NS(=O)(=O)c2ccc(N)cc2)cc1 |r| Show InChI InChI=1S/C28H36N4O6S/c1-3-24-25(23(33)17-38-24)31-27(35)22(16-28(2)14-4-5-15-28)30-26(34)18-6-10-20(11-7-18)32-39(36,37)21-12-8-19(29)9-13-21/h6-13,22,24-25,32H,3-5,14-17,29H2,1-2H3,(H,30,34)(H,31,35)/t22-,24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S expressed in baculovirus by fluorescence assay |
Bioorg Med Chem 17: 1307-24 (2009)
Article DOI: 10.1016/j.bmc.2008.12.020
BindingDB Entry DOI: 10.7270/Q2GX4BD9 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50335276
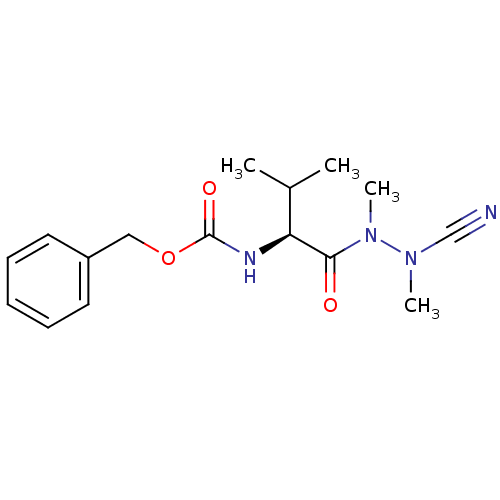
(CHEMBL1651242 | N-(Benzyloxycarbonyl)-valyl-methyl...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C16H22N4O3/c1-12(2)14(15(21)20(4)19(3)11-17)18-16(22)23-10-13-8-6-5-7-9-13/h5-9,12,14H,10H2,1-4H3,(H,18,22)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19778

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CC[C@@H](C)N(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)14-21(30-27(34)24-15-19-8-4-5-9-23(19)37-24)26(33)29-20-12-11-18(3)31(16-22(20)32)38(35,36)25-10-6-7-13-28-25/h4-10,13,15,17-18,20-21H,11-12,14,16H2,1-3H3,(H,29,33)(H,30,34)/t18-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM103364

(US8552202, Compound 13)Show SMILES Cl[C@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1cccc(c1)-n1ccnc1)C1CCCCC1 |r| Show InChI InChI=1S/C24H27ClN4O4/c25-18-12-29(21-19(30)13-33-22(18)21)24(32)20(15-5-2-1-3-6-15)27-23(31)16-7-4-8-17(11-16)28-10-9-26-14-28/h4,7-11,14-15,18,20-22H,1-3,5-6,12-13H2,(H,27,31)/t18-,20-,21+,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50032805

(CHEMBL107495 | N-[2-(3-Morpholin-4-yl-ureido)-1-na...)Show SMILES O=C(NN1CCOCC1)NC(=O)C(Cc1ccc2ccccc2c1)NC(=O)C(CCc1ccccc1)N=CCS(=O)(=O)c1ccc2ccccc2c1 |w:36.39| Show InChI InChI=1S/C40H41N5O6S/c46-38(36(19-15-29-8-2-1-3-9-29)41-20-25-52(49,50)35-18-17-32-11-5-7-13-34(32)28-35)42-37(27-30-14-16-31-10-4-6-12-33(31)26-30)39(47)43-40(48)44-45-21-23-51-24-22-45/h1-14,16-18,20,26,28,36-37H,15,19,21-25,27H2,(H,42,46)(H2,43,44,47,48) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Khepri Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound is evaluated for inhibitory potency against cathepsin S |
J Med Chem 38: 3193-6 (1995)
BindingDB Entry DOI: 10.7270/Q21C1VXJ |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50255949
(4-Ethanesulfonylamino-N-[1-(2S-ethyl-4-oxo-tetrahy...)Show SMILES CC[C@@H]1OCC(=O)[C@H]1NC(=O)[C@H](CC1(C)CCCC1)NC(=O)c1ccc(NS(=O)(=O)CC)cc1 |r| Show InChI InChI=1S/C24H35N3O6S/c1-4-20-21(19(28)15-33-20)26-23(30)18(14-24(3)12-6-7-13-24)25-22(29)16-8-10-17(11-9-16)27-34(31,32)5-2/h8-11,18,20-21,27H,4-7,12-15H2,1-3H3,(H,25,29)(H,26,30)/t18-,20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S expressed in baculovirus by fluorescence assay |
Bioorg Med Chem 17: 1307-24 (2009)
Article DOI: 10.1016/j.bmc.2008.12.020
BindingDB Entry DOI: 10.7270/Q2GX4BD9 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50463221
(CHEMBL4251253)Show SMILES FC(F)(F)Oc1ccc(Nc2nc(nc(n2)N2CCOCC2)C#N)cc1 Show InChI InChI=1S/C15H13F3N6O2/c16-15(17,18)26-11-3-1-10(2-4-11)20-13-21-12(9-19)22-14(23-13)24-5-7-25-8-6-24/h1-4H,5-8H2,(H,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S using Z-LR-AMC as substrate after 30 mins by Cheng-Prusoff equation analysis |
Bioorg Med Chem 26: 4310-4319 (2018)
Article DOI: 10.1016/j.bmc.2018.07.032
BindingDB Entry DOI: 10.7270/Q2P271RF |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19577
((2S)-3-cyclohexyl-2-({4-[(4,6-dimethylpyrimidin-2-...)Show SMILES COc1ccc(NCCNC(=O)[C@H](CC2CCCCC2)NC(=O)c2ccc(Nc3nc(C)cc(C)n3)cc2)cc1 |r| Show InChI InChI=1S/C31H40N6O3/c1-21-19-22(2)35-31(34-21)36-26-11-9-24(10-12-26)29(38)37-28(20-23-7-5-4-6-8-23)30(39)33-18-17-32-25-13-15-27(40-3)16-14-25/h9-16,19,23,28,32H,4-8,17-18,20H2,1-3H3,(H,33,39)(H,37,38)(H,34,35,36)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 15: 4979-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.017
BindingDB Entry DOI: 10.7270/Q2ZG6QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM109857

(US8609681, 94)Show SMILES CCCC(F)(F)C[C@H](NC(=O)N1CCC2(CCN(CC2)C2CC2)CC1)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C24H37F2N5O2/c1-2-5-24(25,26)16-19(20(32)29-23(17-27)6-7-23)28-21(33)31-14-10-22(11-15-31)8-12-30(13-9-22)18-3-4-18/h18-19H,2-16H2,1H3,(H,28,33)(H,29,32)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Determination of the enzymatic activity of the catalytic domain of human Cathepsin S. This protein is obtained as an inactive enzyme from R&D Systems... |
US Patent US8609681 (2013)
BindingDB Entry DOI: 10.7270/Q2F47MSF |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM109856
(US8609681, 91)Show SMILES CCCC(F)(F)C[C@H](NC(=O)N1CCC2(CCN(C2)c2ccc(OC)cc2)CC1)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C27H37F2N5O3/c1-3-8-27(28,29)17-22(23(35)32-26(18-30)9-10-26)31-24(36)33-14-11-25(12-15-33)13-16-34(19-25)20-4-6-21(37-2)7-5-20/h4-7,22H,3,8-17,19H2,1-2H3,(H,31,36)(H,32,35)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Determination of the enzymatic activity of the catalytic domain of human Cathepsin S. This protein is obtained as an inactive enzyme from R&D Systems... |
US Patent US8609681 (2013)
BindingDB Entry DOI: 10.7270/Q2F47MSF |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM109839

(US8609681, 36)Show SMILES CC(F)(F)C[C@H](NC(=O)N1CCC2(CCCC2)CC1)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C19H28F2N4O2/c1-17(20,21)12-14(15(26)24-19(13-22)6-7-19)23-16(27)25-10-8-18(9-11-25)4-2-3-5-18/h14H,2-12H2,1H3,(H,23,27)(H,24,26)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
Determination of the enzymatic activity of the catalytic domain of human Cathepsin S. This protein is obtained as an inactive enzyme from R&D Systems... |
US Patent US8609681 (2013)
BindingDB Entry DOI: 10.7270/Q2F47MSF |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50255456
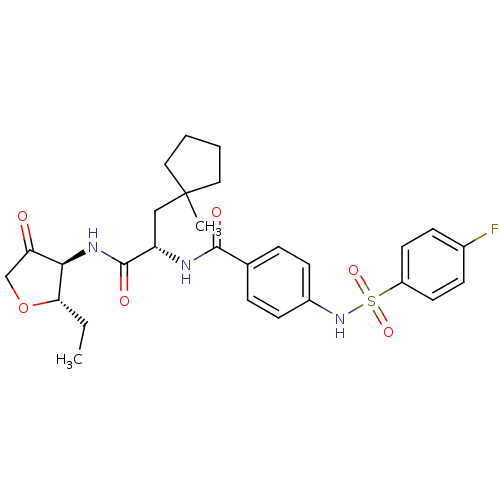
(CHEMBL474664 | N-[1-(2S-Ethyl-4-oxo-tetrahydro-fur...)Show SMILES CC[C@@H]1OCC(=O)[C@H]1NC(=O)[C@H](CC1(C)CCCC1)NC(=O)c1ccc(NS(=O)(=O)c2ccc(F)cc2)cc1 |r| Show InChI InChI=1S/C28H34FN3O6S/c1-3-24-25(23(33)17-38-24)31-27(35)22(16-28(2)14-4-5-15-28)30-26(34)18-6-10-20(11-7-18)32-39(36,37)21-12-8-19(29)9-13-21/h6-13,22,24-25,32H,3-5,14-17H2,1-2H3,(H,30,34)(H,31,35)/t22-,24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S expressed in baculovirus by fluorescence assay |
Bioorg Med Chem 17: 1307-24 (2009)
Article DOI: 10.1016/j.bmc.2008.12.020
BindingDB Entry DOI: 10.7270/Q2GX4BD9 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50255629

(CHEMBL480638 | N-[1-(2S-Ethyl-4-oxo-tetrahydro-fur...)Show SMILES CC[C@@H]1OCC(=O)[C@H]1NC(=O)[C@H](CC1(C)CCCC1)NC(=O)c1ccc(NS(=O)(=O)c2cccnc2)cc1 |r| Show InChI InChI=1S/C27H34N4O6S/c1-3-23-24(22(32)17-37-23)30-26(34)21(15-27(2)12-4-5-13-27)29-25(33)18-8-10-19(11-9-18)31-38(35,36)20-7-6-14-28-16-20/h6-11,14,16,21,23-24,31H,3-5,12-13,15,17H2,1-2H3,(H,29,33)(H,30,34)/t21-,23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S expressed in baculovirus by fluorescence assay |
Bioorg Med Chem 17: 1307-24 (2009)
Article DOI: 10.1016/j.bmc.2008.12.020
BindingDB Entry DOI: 10.7270/Q2GX4BD9 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM103361
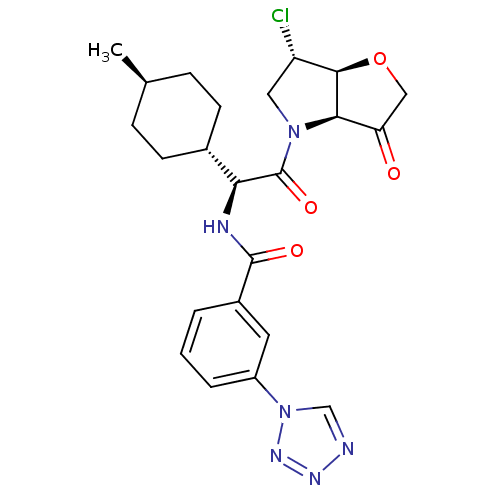
(US8552202, Example 2)Show SMILES C[C@H]1CC[C@@H](CC1)[C@H](NC(=O)c1cccc(c1)-n1cnnn1)C(=O)N1C[C@H](Cl)[C@H]2OCC(=O)[C@@H]12 |r,wU:7.8,33.36,28.31,4.4,wD:26.29,1.0,(1.93,4.62,;1.93,3.08,;.6,2.31,;.6,.77,;1.93,,;3.27,.77,;3.27,2.31,;1.93,-1.54,;.6,-2.31,;-.73,-1.54,;-.73,,;-2.07,-2.31,;-2.07,-3.85,;-3.4,-4.62,;-4.73,-3.85,;-4.73,-2.31,;-3.4,-1.54,;-6.07,-1.54,;-7.53,-2.02,;-8.44,-.77,;-7.53,.48,;-6.07,,;3.27,-2.31,;3.27,-3.85,;4.6,-1.54,;4.6,,;6.07,.48,;6.84,1.81,;6.97,-.77,;8.44,-1.25,;8.44,-2.79,;6.97,-3.26,;6.2,-4.6,;6.07,-2.02,)| Show InChI InChI=1S/C23H27ClN6O4/c1-13-5-7-14(8-6-13)19(23(33)29-10-17(24)21-20(29)18(31)11-34-21)26-22(32)15-3-2-4-16(9-15)30-12-25-27-28-30/h2-4,9,12-14,17,19-21H,5-8,10-11H2,1H3,(H,26,32)/t13-,14-,17-,19-,20+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data