

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydroorotate dehydrogenase (fumarate) (Saccharomyces cerevisiae) | BDBM92604 (DHOD Inhibitor, 3 | US8703811, 1) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (fumarate) (Saccharomyces cerevisiae) | BDBM92602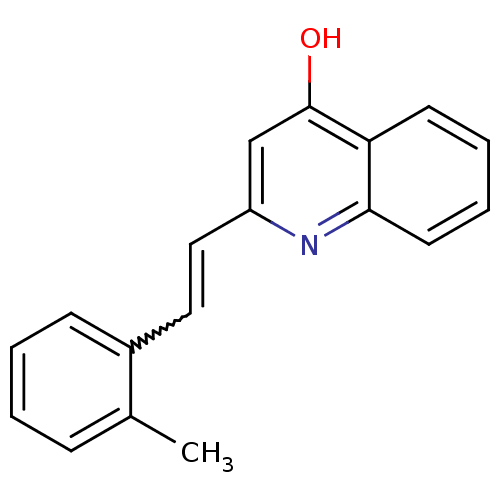 (DHOD Inhibitor, 1 | US8703811, 2) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (fumarate) (Saccharomyces cerevisiae) | BDBM92603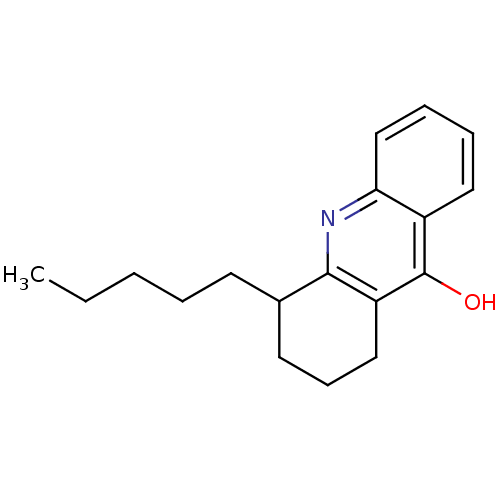 (DHOD Inhibitor, 2 | US8703811, 3) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (fumarate) (Saccharomyces cerevisiae) | BDBM120364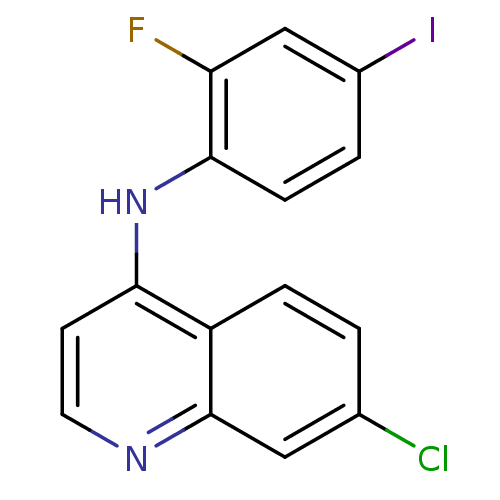 (US8703811, 4) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (fumarate) (Saccharomyces cerevisiae) | BDBM92605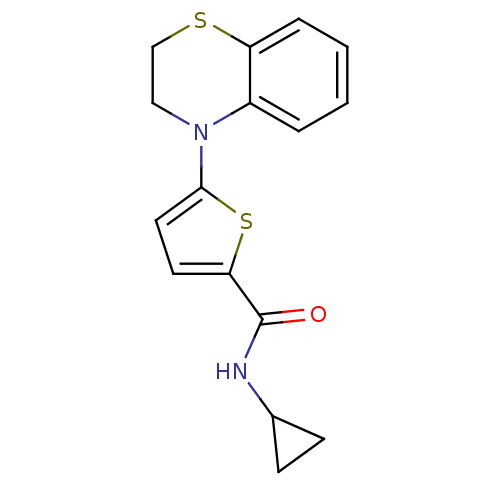 (DHOD Inhibitor, 4 | US8703811, 5) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (fumarate) (Saccharomyces cerevisiae) | BDBM92606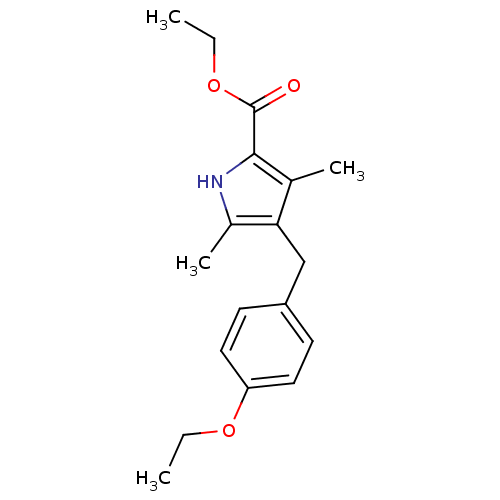 (DHOD Inhibitor, 5 | US8703811, 6) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (fumarate) (Saccharomyces cerevisiae) | BDBM36349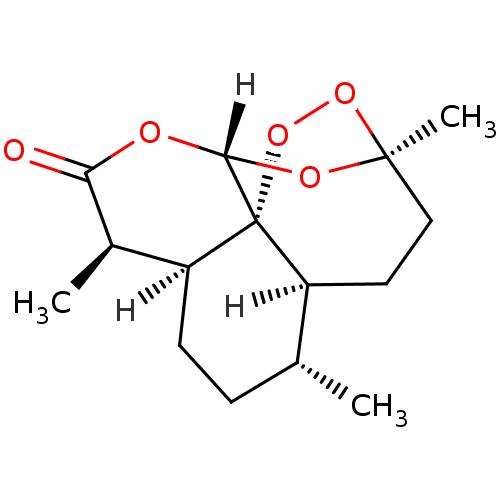 (Artemisinin, 18 | CID452191) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard School of Public Health Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae DHOD | Bioorg Med Chem Lett 19: 972-5 (2009) Article DOI: 10.1016/j.bmcl.2008.11.071 BindingDB Entry DOI: 10.7270/Q2Z89DBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (fumarate) (Saccharomyces cerevisiae) | BDBM50028962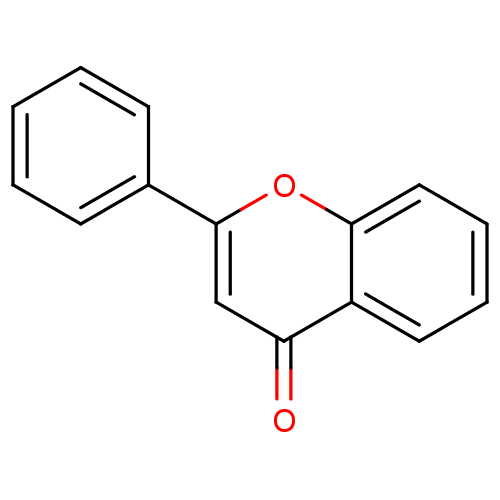 (CHEMBL275638 | flavone) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard School of Public Health Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae DHOD | Bioorg Med Chem Lett 19: 972-5 (2009) Article DOI: 10.1016/j.bmcl.2008.11.071 BindingDB Entry DOI: 10.7270/Q2Z89DBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (fumarate) (Saccharomyces cerevisiae) | BDBM50135527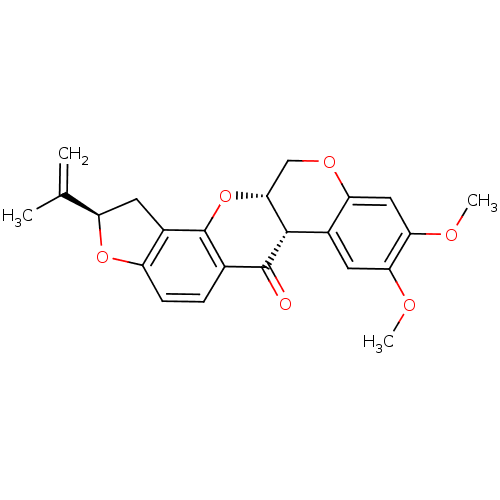 ((-)-cis-rotenone | (-)-rotenone | (2R,6aS,12aS)-8,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard School of Public Health Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae DHOD | Bioorg Med Chem Lett 19: 972-5 (2009) Article DOI: 10.1016/j.bmcl.2008.11.071 BindingDB Entry DOI: 10.7270/Q2Z89DBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (fumarate) (Saccharomyces cerevisiae) | BDBM50256529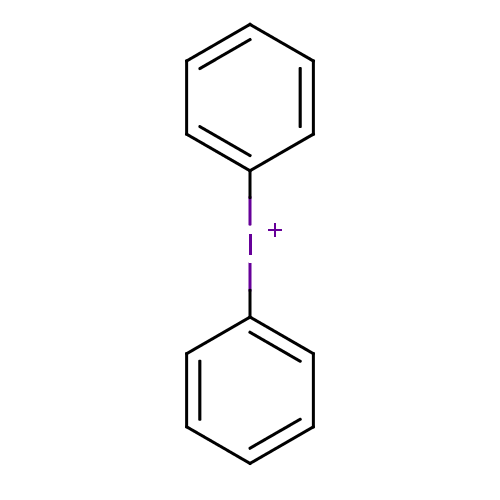 (CHEMBL481680 | diphenyliodonium chloride) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard School of Public Health Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae DHOD | Bioorg Med Chem Lett 19: 972-5 (2009) Article DOI: 10.1016/j.bmcl.2008.11.071 BindingDB Entry DOI: 10.7270/Q2Z89DBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (fumarate) (Saccharomyces cerevisiae) | BDBM50206334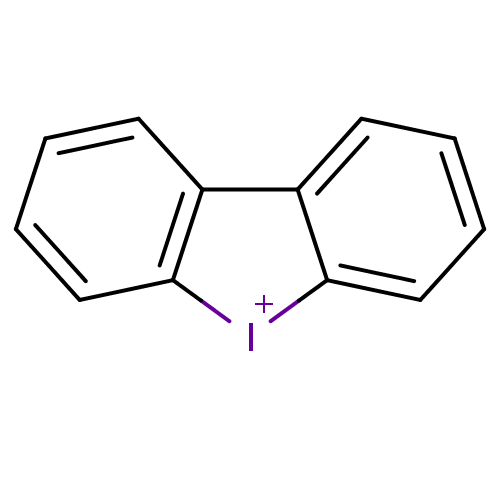 (CHEMBL397686 | Diphenylene iodonium chloride | dib...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard School of Public Health Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae DHOD | Bioorg Med Chem Lett 19: 972-5 (2009) Article DOI: 10.1016/j.bmcl.2008.11.071 BindingDB Entry DOI: 10.7270/Q2Z89DBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (fumarate) (Saccharomyces cerevisiae) | BDBM50203194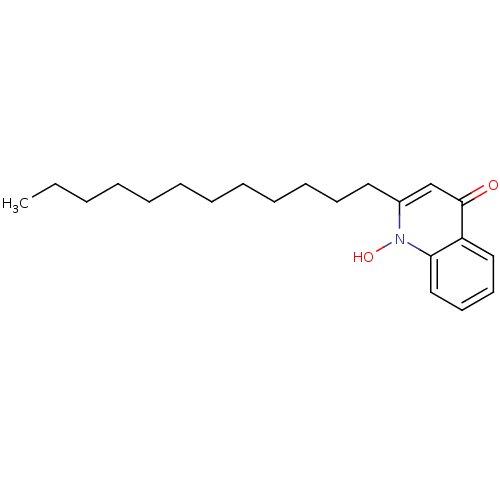 (1-Hydroxy-2-dodecyl-4(1H)quinoline | 1-hydroxy-2-d...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard School of Public Health Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae DHOD | Bioorg Med Chem Lett 19: 972-5 (2009) Article DOI: 10.1016/j.bmcl.2008.11.071 BindingDB Entry DOI: 10.7270/Q2Z89DBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (fumarate) (Saccharomyces cerevisiae) | BDBM50191588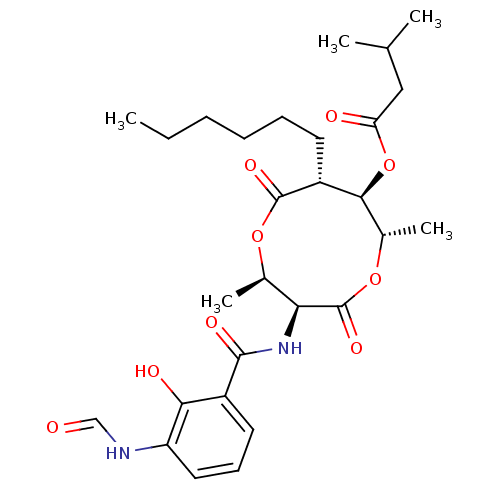 ((2R,3S,6S,7R,8R)-3-[(3-formamido-2-hydroxybenzoyl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard School of Public Health Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae DHOD | Bioorg Med Chem Lett 19: 972-5 (2009) Article DOI: 10.1016/j.bmcl.2008.11.071 BindingDB Entry DOI: 10.7270/Q2Z89DBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (fumarate) (Saccharomyces cerevisiae) | BDBM50203188 (2-((1r,4r)-4-(4-chlorophenyl)cyclohexyl)-3-hydroxy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard School of Public Health Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae DHOD | Bioorg Med Chem Lett 19: 972-5 (2009) Article DOI: 10.1016/j.bmcl.2008.11.071 BindingDB Entry DOI: 10.7270/Q2Z89DBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||