

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50231346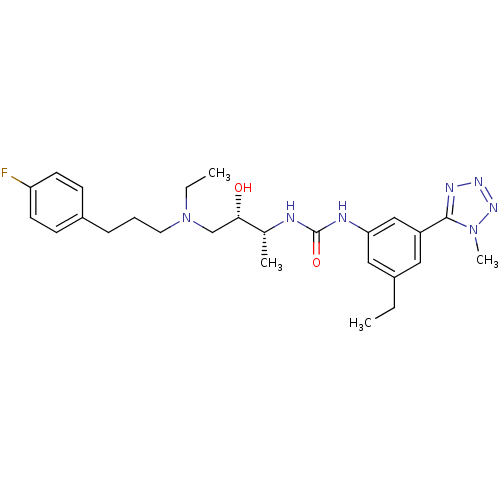 (1-((2R,3S)-4-(ethyl(3-(4-fluorophenyl)propyl)amino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor assessed as inhibition of chemotaxis in eosinophil | Bioorg Med Chem Lett 18: 586-95 (2008) Article DOI: 10.1016/j.bmcl.2007.11.087 BindingDB Entry DOI: 10.7270/Q2FF3S4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50231377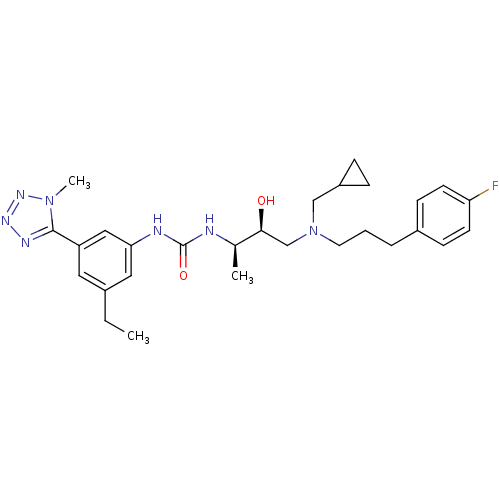 (1-((2R,3S)-4-((cyclopropylmethyl)(3-(4-fluoropheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor assessed as inhibition of chemotaxis in eosinophil | Bioorg Med Chem Lett 18: 586-95 (2008) Article DOI: 10.1016/j.bmcl.2007.11.087 BindingDB Entry DOI: 10.7270/Q2FF3S4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163650 (1-(3,5-Diacetyl-phenyl)-3-{(1R,2S)-2-[(S)-3-(4-flu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163634 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor assessed as inhibition of chemotaxis in eosinophil at 30 nM | Bioorg Med Chem Lett 18: 586-95 (2008) Article DOI: 10.1016/j.bmcl.2007.11.087 BindingDB Entry DOI: 10.7270/Q2FF3S4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163634 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor assessed as inhibition of chemotaxis in eosinophil | Bioorg Med Chem Lett 18: 586-95 (2008) Article DOI: 10.1016/j.bmcl.2007.11.087 BindingDB Entry DOI: 10.7270/Q2FF3S4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163634 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor in human eosinophil assessed as inhibition of eotaxin-induced chemotaxis | Bioorg Med Chem Lett 18: 576-85 (2008) Article DOI: 10.1016/j.bmcl.2007.11.067 BindingDB Entry DOI: 10.7270/Q2057GS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163634 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163634 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at CCR3 assessed as eotaxin-induced chemotaxis in human eosinophils | Bioorg Med Chem Lett 17: 2992-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.065 BindingDB Entry DOI: 10.7270/Q2V987SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50372859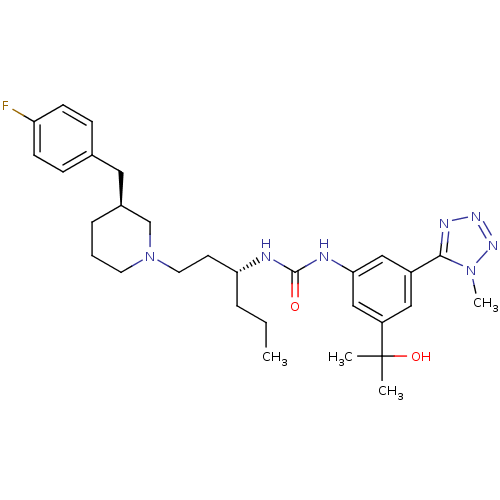 (CHEMBL257074) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor in human eosinophil assessed as inhibition of eotaxin-induced chemotaxis | Bioorg Med Chem Lett 18: 576-85 (2008) Article DOI: 10.1016/j.bmcl.2007.11.067 BindingDB Entry DOI: 10.7270/Q2057GS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50372839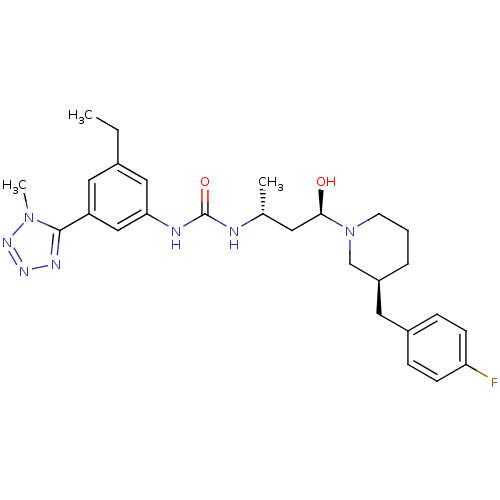 (CHEMBL437031) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor in human eosinophil assessed as inhibition of eotaxin-induced chemotaxis | Bioorg Med Chem Lett 18: 576-85 (2008) Article DOI: 10.1016/j.bmcl.2007.11.067 BindingDB Entry DOI: 10.7270/Q2057GS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50231350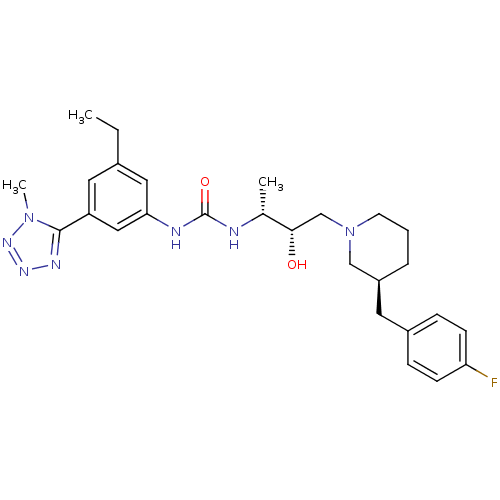 (1-((2R,3S)-4-(ethyl(3-(4-fluorophenyl)propyl)amino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor assessed as inhibition of chemotaxis in eosinophil | Bioorg Med Chem Lett 18: 586-95 (2008) Article DOI: 10.1016/j.bmcl.2007.11.087 BindingDB Entry DOI: 10.7270/Q2FF3S4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163661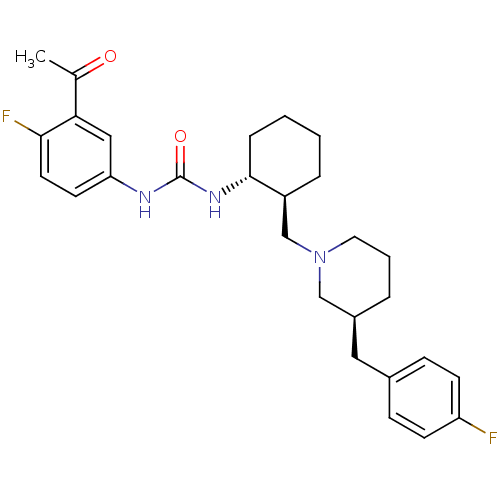 (1-(3-Acetyl-4-fluoro-phenyl)-3-{(1R,2S)-2-[(S)-3-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50410314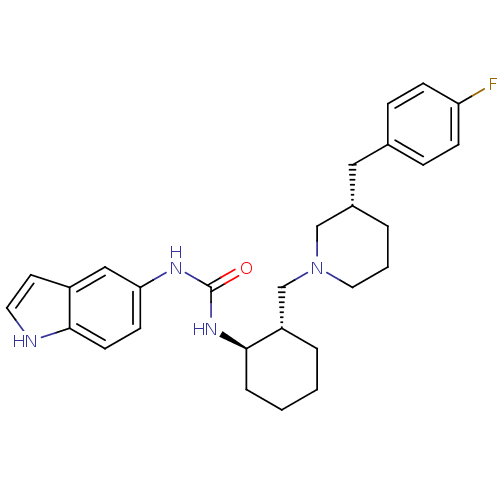 (CHEMBL2113074) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50372854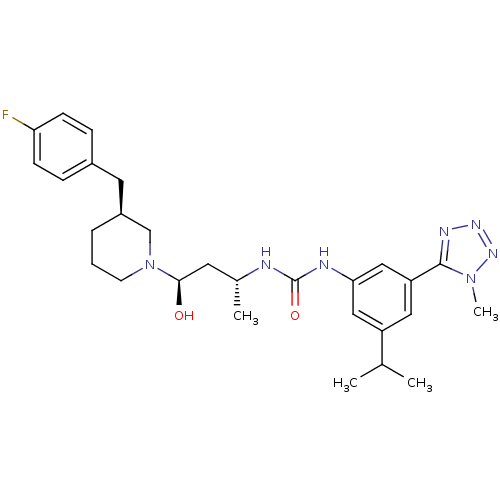 (CHEMBL257293) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor in human eosinophil assessed as inhibition of eotaxin-induced chemotaxis | Bioorg Med Chem Lett 18: 576-85 (2008) Article DOI: 10.1016/j.bmcl.2007.11.067 BindingDB Entry DOI: 10.7270/Q2057GS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50372861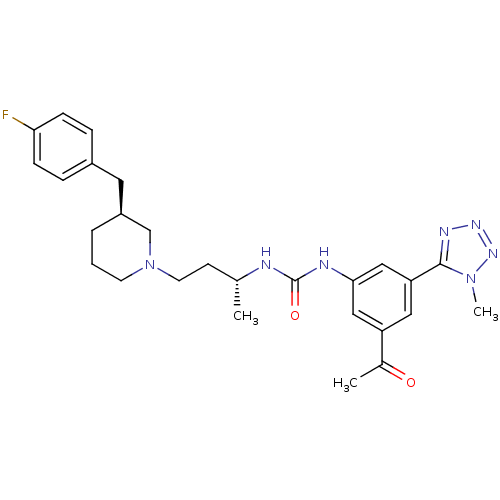 (CHEMBL270582) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor in human eosinophil assessed as inhibition of eotaxin-induced chemotaxis | Bioorg Med Chem Lett 18: 576-85 (2008) Article DOI: 10.1016/j.bmcl.2007.11.067 BindingDB Entry DOI: 10.7270/Q2057GS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50372833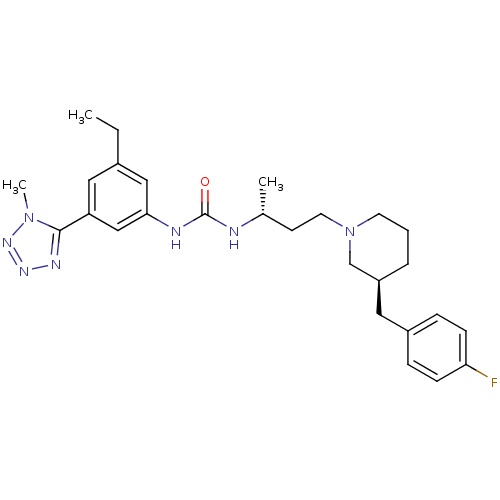 (CHEMBL427728) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor in human eosinophil assessed as inhibition of eotaxin-induced chemotaxis | Bioorg Med Chem Lett 18: 576-85 (2008) Article DOI: 10.1016/j.bmcl.2007.11.067 BindingDB Entry DOI: 10.7270/Q2057GS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50372842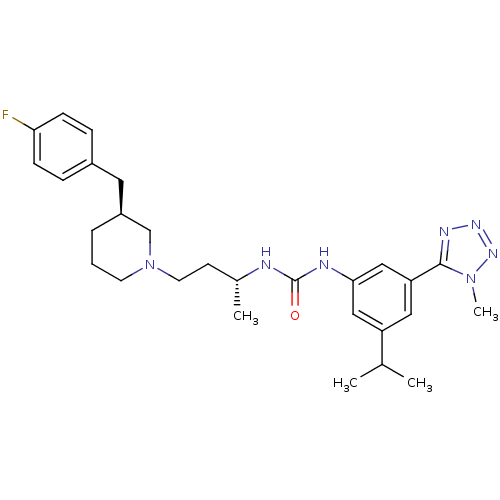 (CHEMBL404122) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor in human eosinophil assessed as inhibition of eotaxin-induced chemotaxis | Bioorg Med Chem Lett 18: 576-85 (2008) Article DOI: 10.1016/j.bmcl.2007.11.067 BindingDB Entry DOI: 10.7270/Q2057GS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163636 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at CCR3 assessed as eotaxin-induced chemotaxis in human eosinophils | Bioorg Med Chem Lett 17: 2992-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.065 BindingDB Entry DOI: 10.7270/Q2V987SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163636 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209971 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at CCR3 assessed as eotaxin-induced chemotaxis in human eosinophils | Bioorg Med Chem Lett 17: 2992-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.065 BindingDB Entry DOI: 10.7270/Q2V987SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209971 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor in human eosinophil assessed as inhibition of eotaxin-induced chemotaxis | Bioorg Med Chem Lett 18: 576-85 (2008) Article DOI: 10.1016/j.bmcl.2007.11.067 BindingDB Entry DOI: 10.7270/Q2057GS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209971 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute Curated by ChEMBL | Assay Description Antagonist activity at CCR3 assessed as inhibition of chemotaxis by cell based assay | J Med Chem 55: 9363-92 (2012) Article DOI: 10.1021/jm300682j BindingDB Entry DOI: 10.7270/Q2862HKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209971 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50231358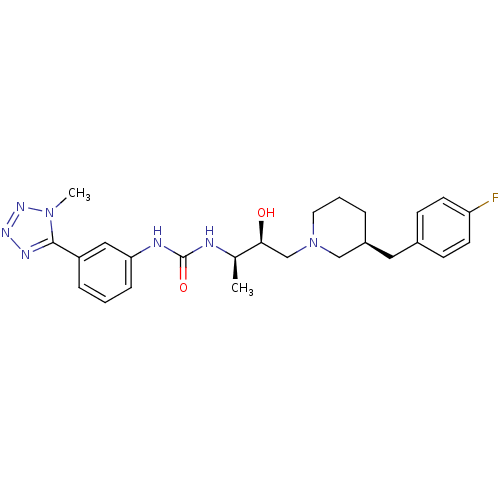 (1-((2R,3S)-4-((S)-3-(4-fluorobenzyl)piperidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor assessed as inhibition of chemotaxis in eosinophil | Bioorg Med Chem Lett 18: 586-95 (2008) Article DOI: 10.1016/j.bmcl.2007.11.087 BindingDB Entry DOI: 10.7270/Q2FF3S4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209971 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of Cynomolgus monkey eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50231358 (1-((2R,3S)-4-((S)-3-(4-fluorobenzyl)piperidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor in human eosinophil assessed as inhibition of eotaxin-induced chemotaxis | Bioorg Med Chem Lett 18: 576-85 (2008) Article DOI: 10.1016/j.bmcl.2007.11.067 BindingDB Entry DOI: 10.7270/Q2057GS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163678 (1-[3,5-Bis-(1-methyl-1H-tetrazol-5-yl)-phenyl]-3-{...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50410322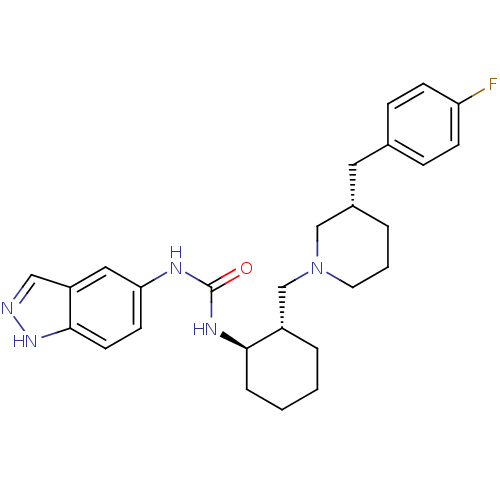 (CHEMBL2113077) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50372855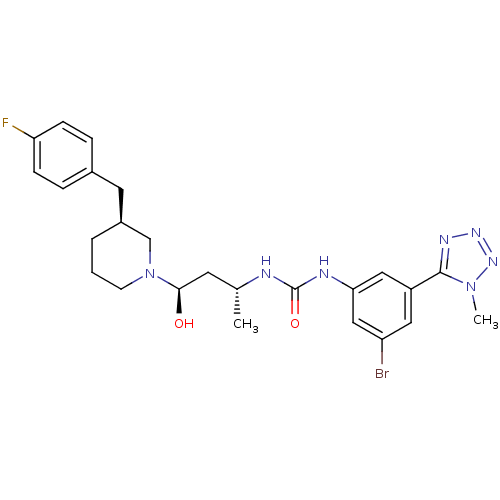 (CHEMBL402983) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor in human eosinophil assessed as inhibition of eotaxin-induced chemotaxis | Bioorg Med Chem Lett 18: 576-85 (2008) Article DOI: 10.1016/j.bmcl.2007.11.067 BindingDB Entry DOI: 10.7270/Q2057GS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50372826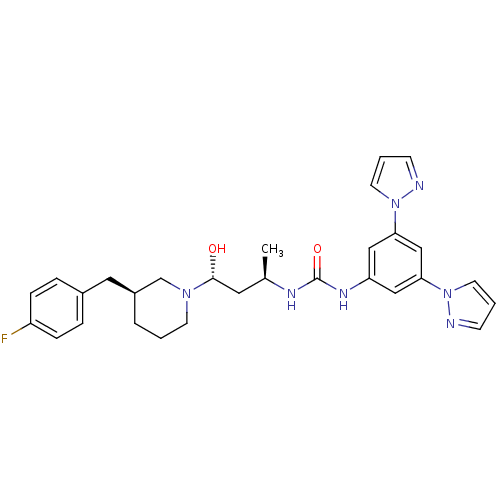 (CHEMBL401879) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor in human eosinophil assessed as inhibition of eotaxin-induced chemotaxis | Bioorg Med Chem Lett 18: 576-85 (2008) Article DOI: 10.1016/j.bmcl.2007.11.067 BindingDB Entry DOI: 10.7270/Q2057GS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163662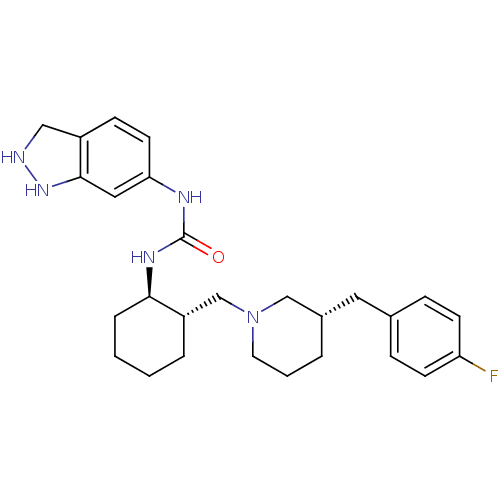 (1-(3a,7a-Dihydro-1H-indazol-6-yl)-3-{(1R,2S)-2-[(S...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50372857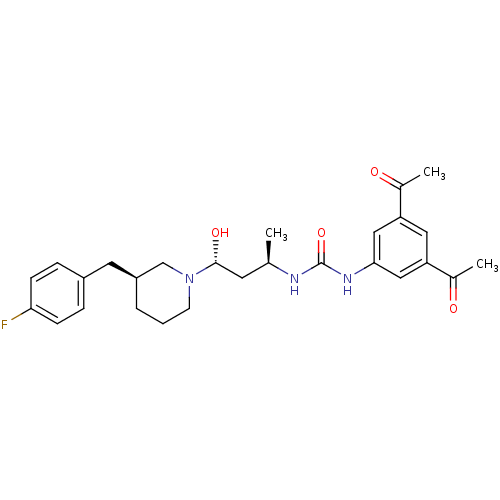 (CHEMBL256068) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor in human eosinophil assessed as inhibition of eotaxin-induced chemotaxis | Bioorg Med Chem Lett 18: 576-85 (2008) Article DOI: 10.1016/j.bmcl.2007.11.067 BindingDB Entry DOI: 10.7270/Q2057GS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50372835 (CHEMBL258156) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor in human eosinophil assessed as inhibition of eotaxin-induced chemotaxis | Bioorg Med Chem Lett 18: 576-85 (2008) Article DOI: 10.1016/j.bmcl.2007.11.067 BindingDB Entry DOI: 10.7270/Q2057GS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209974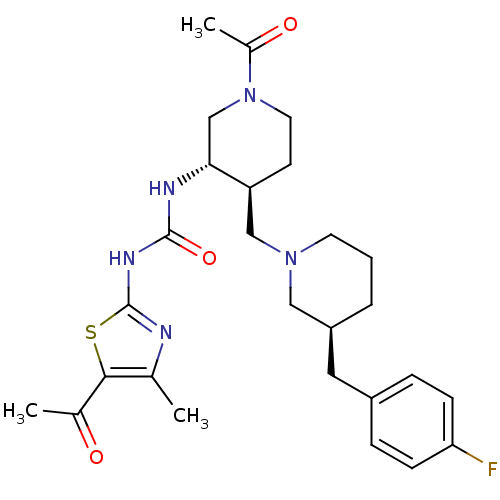 (1-((3S,4S)-4-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at CCR3 assessed as eotaxin-induced chemotaxis in human eosinophils | Bioorg Med Chem Lett 17: 2992-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.065 BindingDB Entry DOI: 10.7270/Q2V987SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209972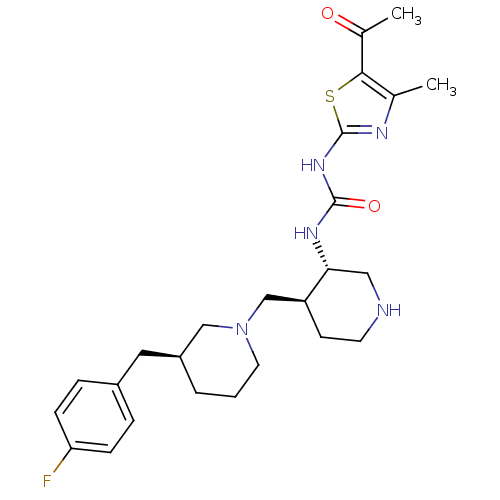 (1-((3S,4S)-4-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at CCR3 assessed as eotaxin-induced chemotaxis in human eosinophils | Bioorg Med Chem Lett 17: 2992-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.065 BindingDB Entry DOI: 10.7270/Q2V987SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163660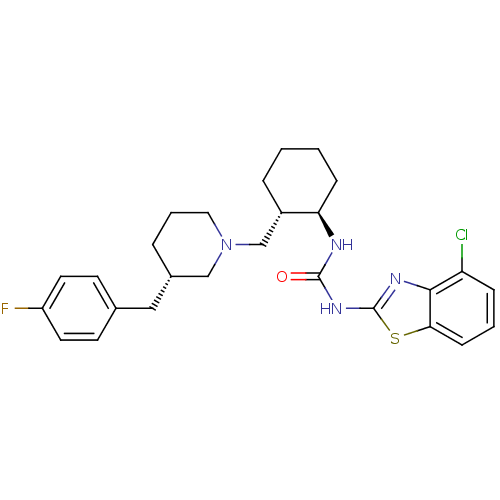 (1-(4-Chloro-benzothiazol-2-yl)-3-{(1R,2S)-2-[(S)-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163656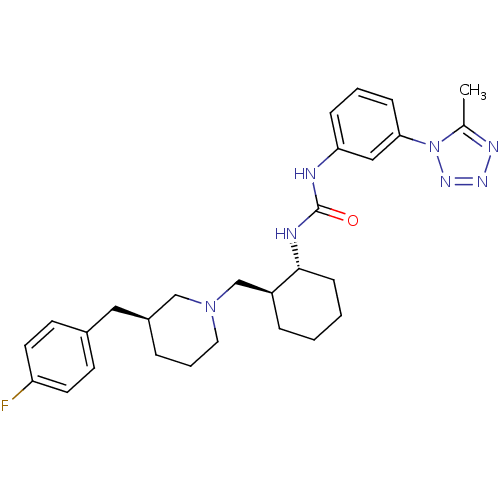 (1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50372837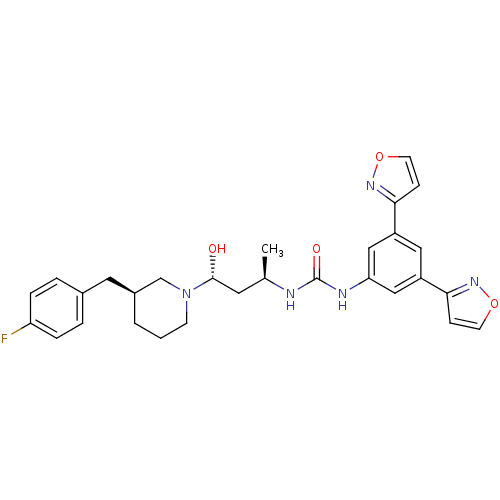 (CHEMBL429846) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor in human eosinophil assessed as inhibition of eotaxin-induced chemotaxis | Bioorg Med Chem Lett 18: 576-85 (2008) Article DOI: 10.1016/j.bmcl.2007.11.067 BindingDB Entry DOI: 10.7270/Q2057GS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209989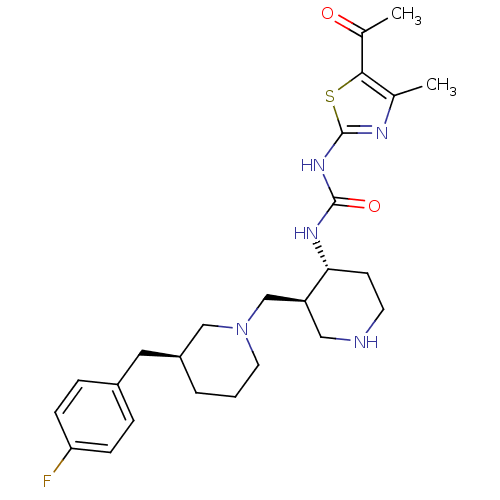 (1-((3S,4R)-3-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at CCR3 assessed as eotaxin-induced chemotaxis in human eosinophils | Bioorg Med Chem Lett 17: 2992-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.065 BindingDB Entry DOI: 10.7270/Q2V987SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50372860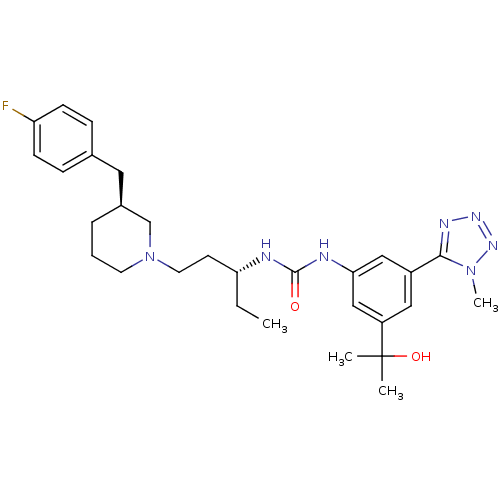 (CHEMBL270146) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor in human eosinophil assessed as inhibition of eotaxin-induced chemotaxis | Bioorg Med Chem Lett 18: 576-85 (2008) Article DOI: 10.1016/j.bmcl.2007.11.067 BindingDB Entry DOI: 10.7270/Q2057GS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50372830 (CHEMBL270147) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor in human eosinophil assessed as inhibition of eotaxin-induced chemotaxis | Bioorg Med Chem Lett 18: 576-85 (2008) Article DOI: 10.1016/j.bmcl.2007.11.067 BindingDB Entry DOI: 10.7270/Q2057GS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50255040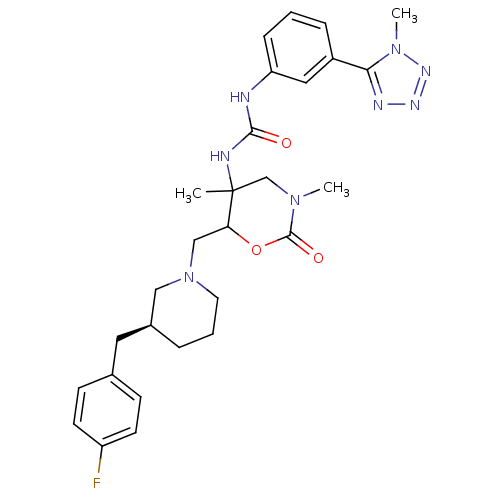 (1-(6-(((S)-3-(4-fluorobenzyl) piperidin-1-yl)methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity to CCR3 receptor (unknown origin) | Bioorg Med Chem Lett 19: 96-9 (2008) Article DOI: 10.1016/j.bmcl.2008.11.002 BindingDB Entry DOI: 10.7270/Q2MG7PCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50255040 (1-(6-(((S)-3-(4-fluorobenzyl) piperidin-1-yl)methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity to CCR3 receptor (unknown origin) | Bioorg Med Chem Lett 19: 96-9 (2008) Article DOI: 10.1016/j.bmcl.2008.11.002 BindingDB Entry DOI: 10.7270/Q2MG7PCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50231371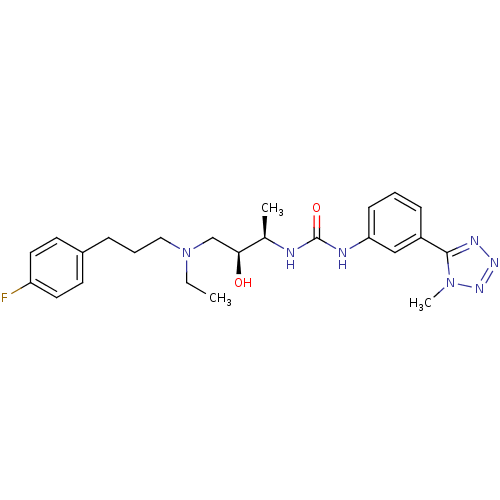 (1-((2R,3S)-4-(ethyl(3-(4-fluorophenyl)propyl)amino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I] eotaxin from human CCR3 receptor in CHO cells | Bioorg Med Chem Lett 18: 586-95 (2008) Article DOI: 10.1016/j.bmcl.2007.11.087 BindingDB Entry DOI: 10.7270/Q2FF3S4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50372829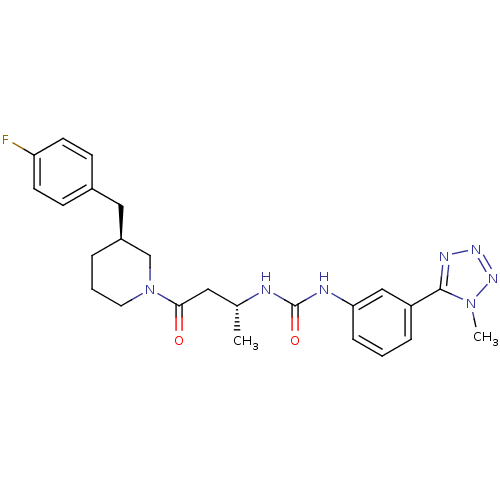 (CHEMBL428071) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor in human eosinophil assessed as inhibition of eotaxin-induced chemotaxis | Bioorg Med Chem Lett 18: 576-85 (2008) Article DOI: 10.1016/j.bmcl.2007.11.067 BindingDB Entry DOI: 10.7270/Q2057GS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50372862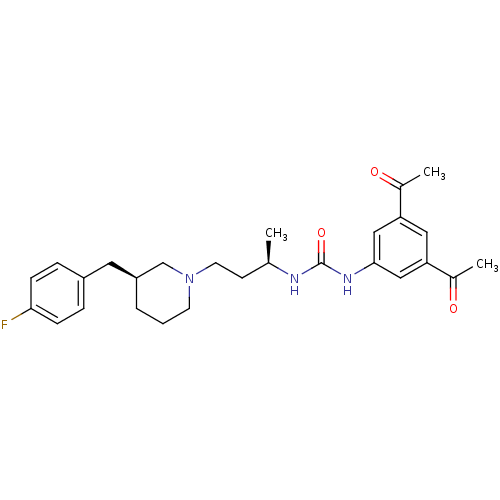 (CHEMBL255398) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor in human eosinophil assessed as inhibition of eotaxin-induced chemotaxis | Bioorg Med Chem Lett 18: 576-85 (2008) Article DOI: 10.1016/j.bmcl.2007.11.067 BindingDB Entry DOI: 10.7270/Q2057GS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50231354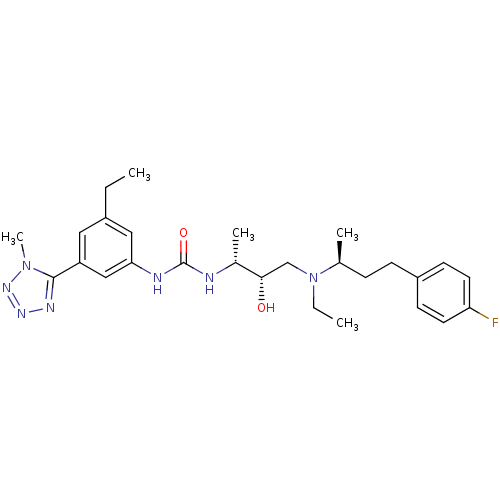 (1-((2R,3S)-4-(ethyl((S)-4-(4-fluorophenyl)butan-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I] eotaxin from human CCR3 receptor in CHO cells | Bioorg Med Chem Lett 18: 586-95 (2008) Article DOI: 10.1016/j.bmcl.2007.11.087 BindingDB Entry DOI: 10.7270/Q2FF3S4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50231360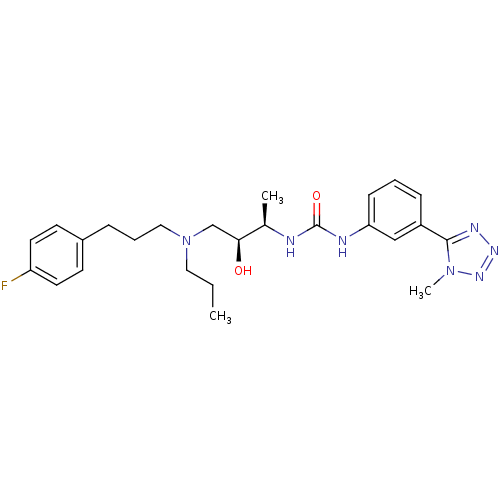 (1-((2R,3S)-4-((3-(4-fluorophenyl)propyl)(propyl)am...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I] eotaxin from human CCR3 receptor in CHO cells | Bioorg Med Chem Lett 18: 586-95 (2008) Article DOI: 10.1016/j.bmcl.2007.11.087 BindingDB Entry DOI: 10.7270/Q2FF3S4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50255006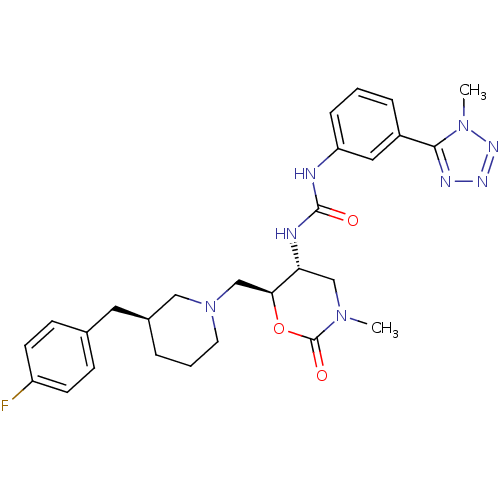 (1-((5R,6S)-6-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity to CCR3 receptor (unknown origin) | Bioorg Med Chem Lett 19: 96-9 (2008) Article DOI: 10.1016/j.bmcl.2008.11.002 BindingDB Entry DOI: 10.7270/Q2MG7PCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163635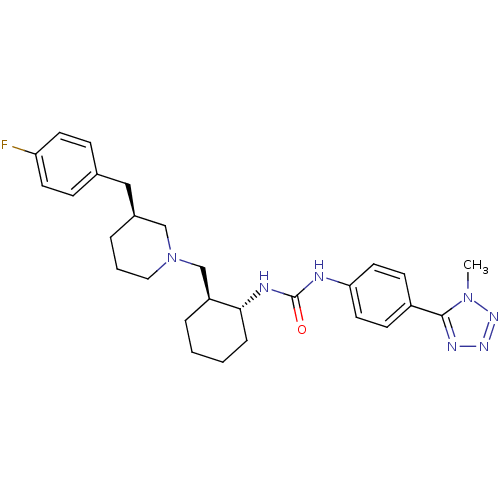 (1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 938 total ) | Next | Last >> |