

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123511 (US8748435, 36) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123488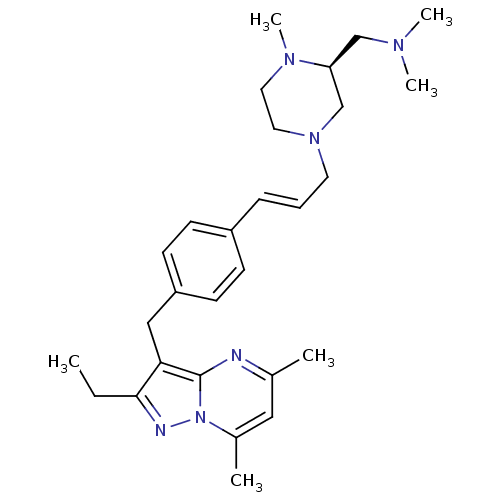 (US8748435, 13) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123489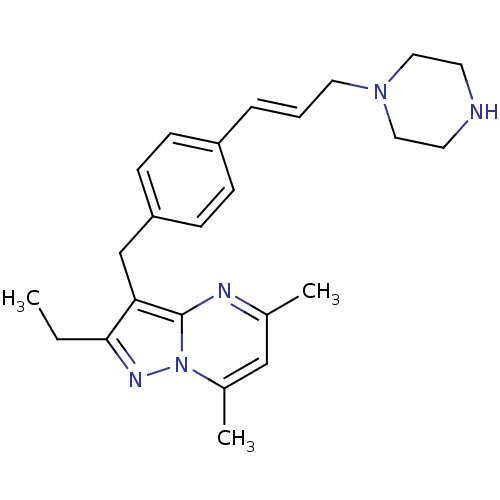 (US8748435, 14) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123514 (US8748435, 39) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123513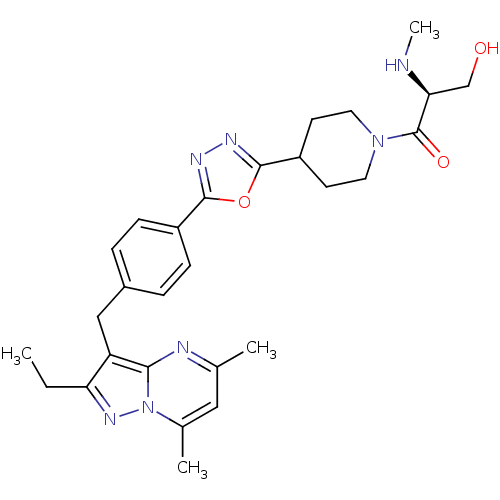 (US8748435, 38) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123480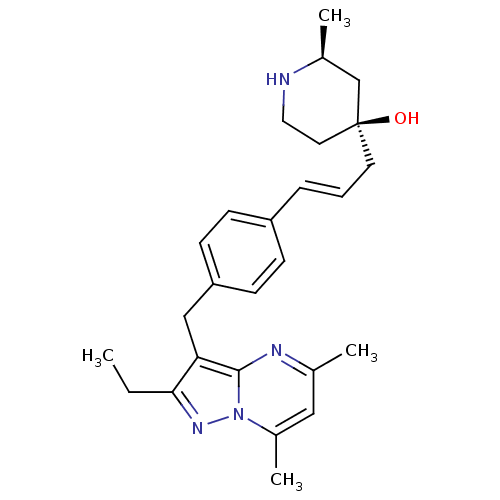 (US8748435, 5) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123497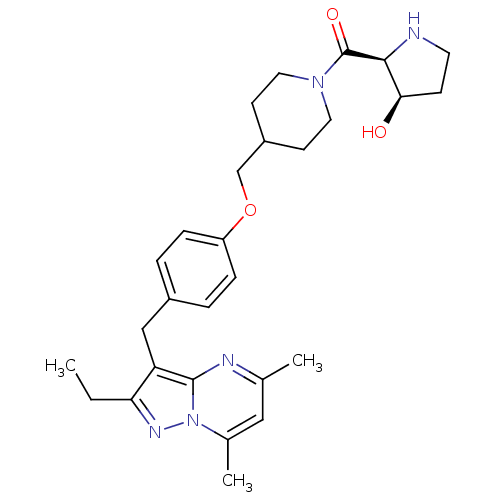 (US8748435, 22) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123484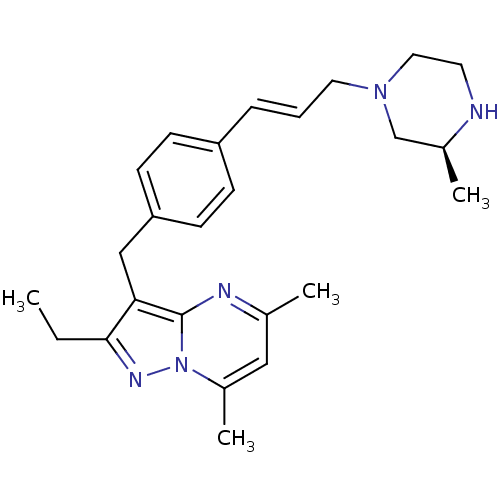 (US8748435, 9) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123514 (US8748435, 39) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human GPR4 expressed in human HeLa cells assessed as inhibition of IBMX-induced cAMP accumulation after 15 mins by HTRF assay | J Med Chem 60: 3672-3683 (2017) Article DOI: 10.1021/acs.jmedchem.6b01703 BindingDB Entry DOI: 10.7270/Q28P6349 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM50450950 (CHEMBL4217603) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human GPR4 expressed in human HeLa cells assessed as inhibition of IBMX-induced cAMP accumulation after 15 mins by HTRF assay | J Med Chem 60: 3672-3683 (2017) Article DOI: 10.1021/acs.jmedchem.6b01703 BindingDB Entry DOI: 10.7270/Q28P6349 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123504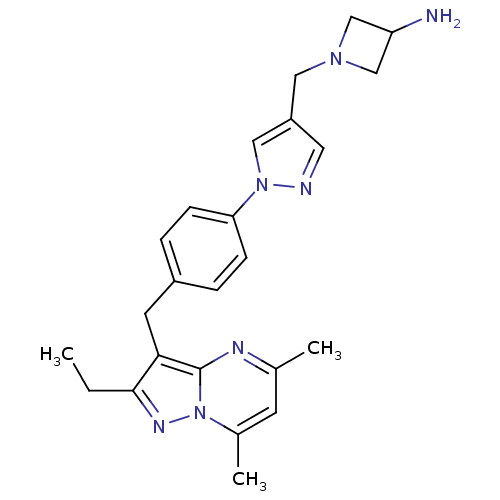 (US8748435, 29) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123496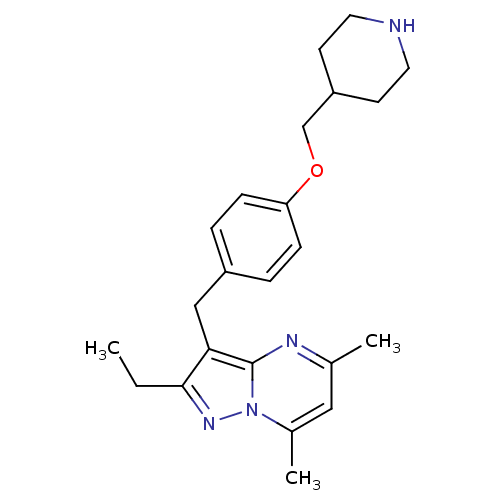 (US8748435, 21) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123481 (US8748435, 6) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM50450954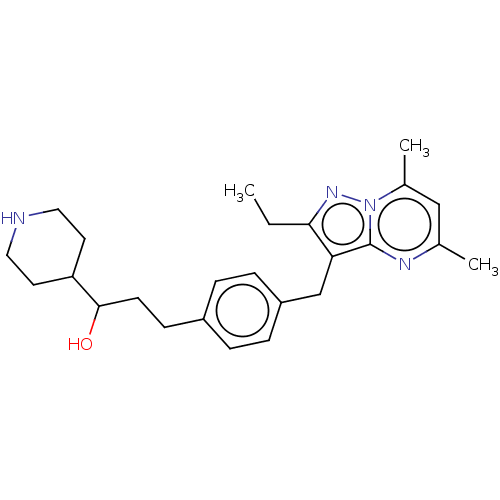 (CHEMBL4208267) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human GPR4 expressed in human HeLa cells assessed as inhibition of IBMX-induced cAMP accumulation after 15 mins by HTRF assay | J Med Chem 60: 3672-3683 (2017) Article DOI: 10.1021/acs.jmedchem.6b01703 BindingDB Entry DOI: 10.7270/Q28P6349 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123505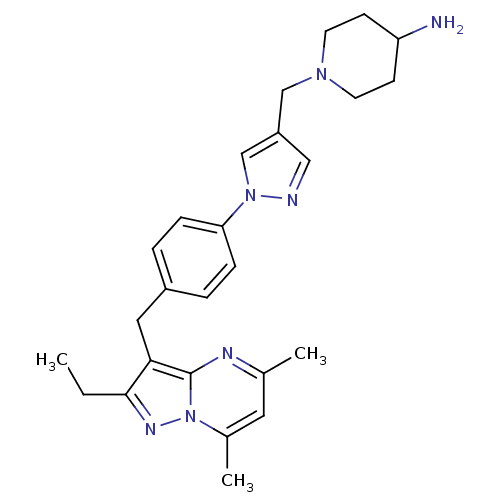 (US8748435, 30) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM50450947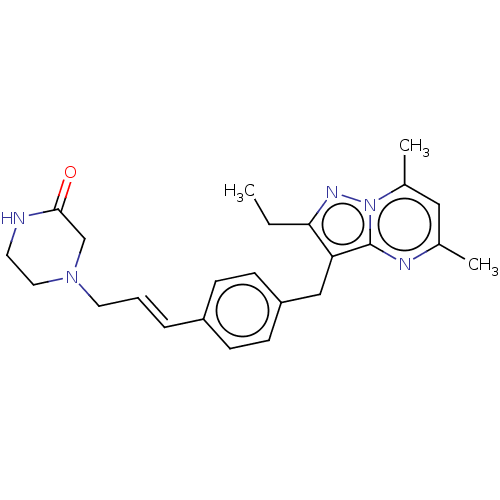 (CHEMBL4210921) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human GPR4 expressed in human HeLa cells assessed as inhibition of IBMX-induced cAMP accumulation after 15 mins by HTRF assay | J Med Chem 60: 3672-3683 (2017) Article DOI: 10.1021/acs.jmedchem.6b01703 BindingDB Entry DOI: 10.7270/Q28P6349 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123483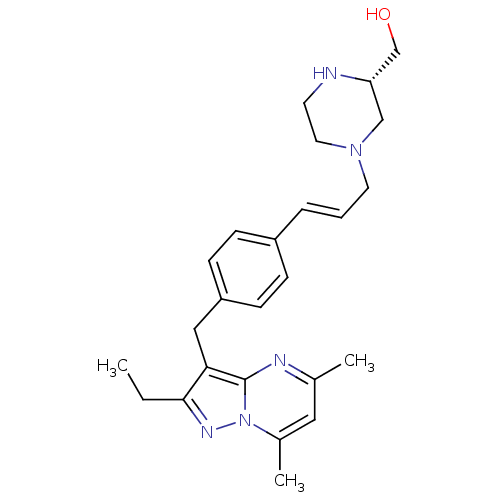 (US8748435, 8) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123492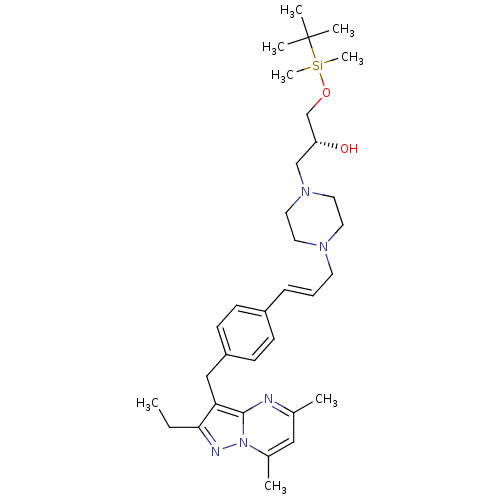 (US8748435, 17) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123485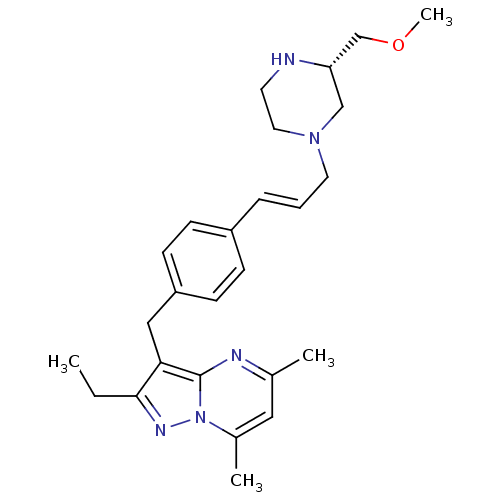 (US8748435, 10) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 33 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123487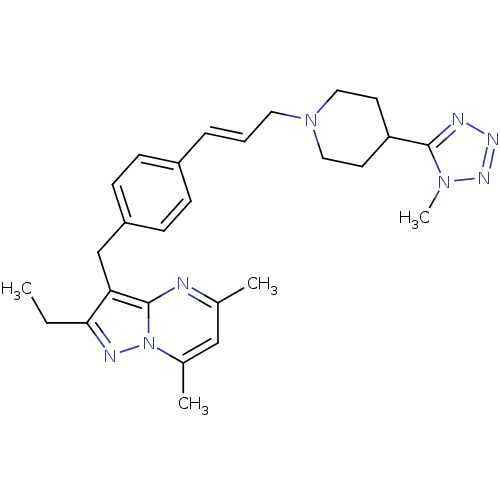 (US8748435, 12) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 33 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123482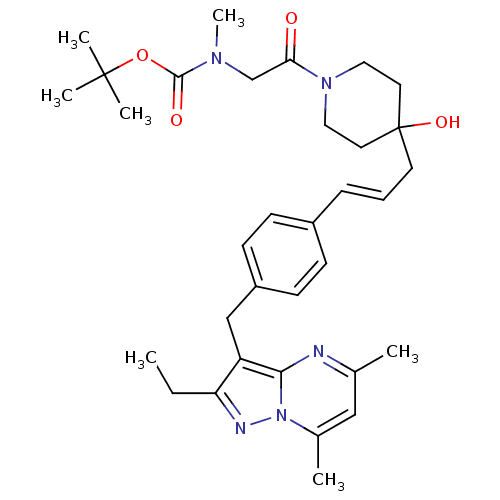 (US8748435, 7) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123477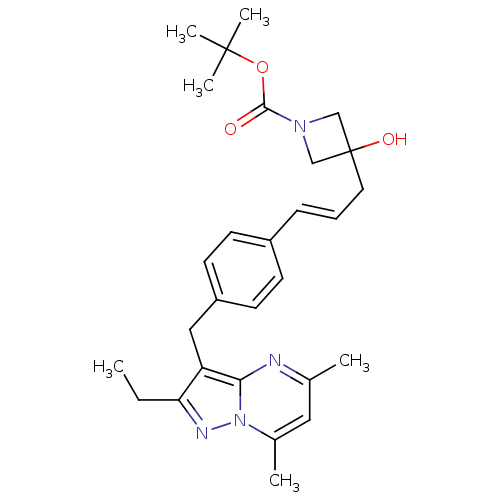 (US8748435, 2) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123498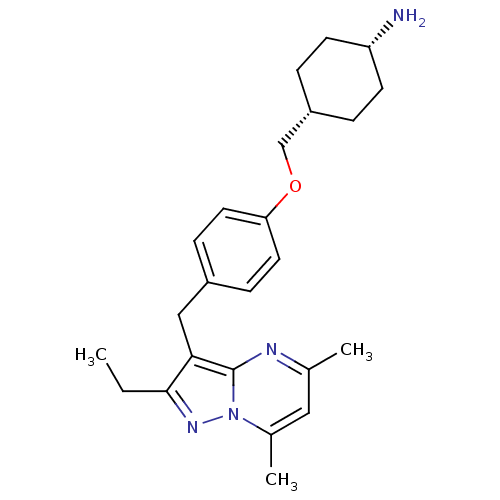 (US8748435, 23) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123491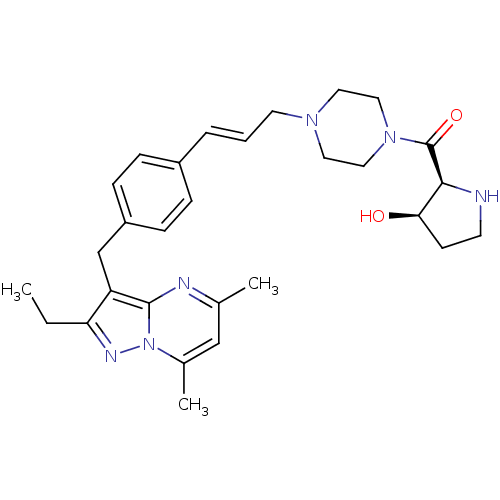 (US8748435, 16) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123486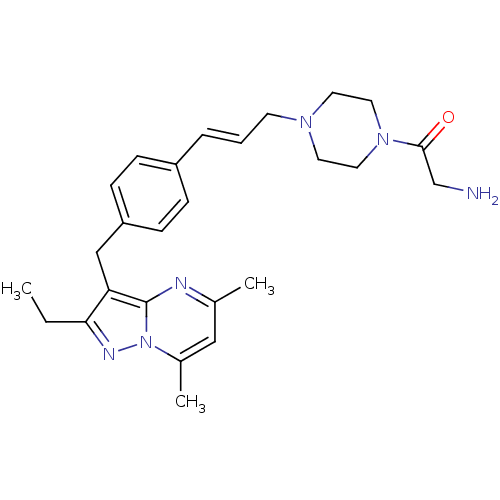 (US8748435, 11) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 38 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123508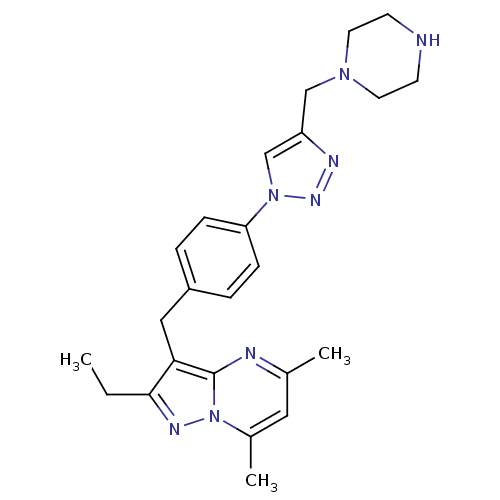 (US8748435, 33) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 38 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM50450953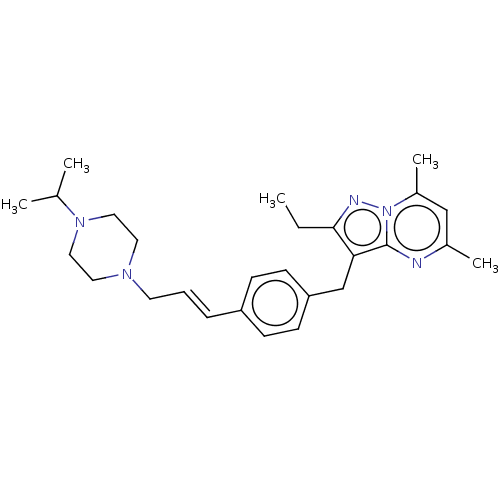 (CHEMBL4209248) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human GPR4 expressed in human HeLa cells assessed as inhibition of IBMX-induced cAMP accumulation after 15 mins by HTRF assay | J Med Chem 60: 3672-3683 (2017) Article DOI: 10.1021/acs.jmedchem.6b01703 BindingDB Entry DOI: 10.7270/Q28P6349 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123506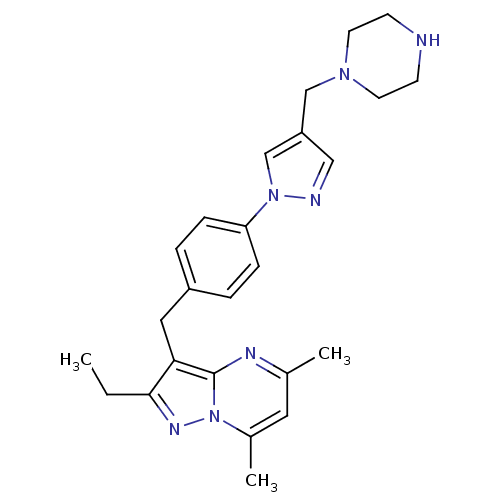 (US8748435, 31) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 43 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123501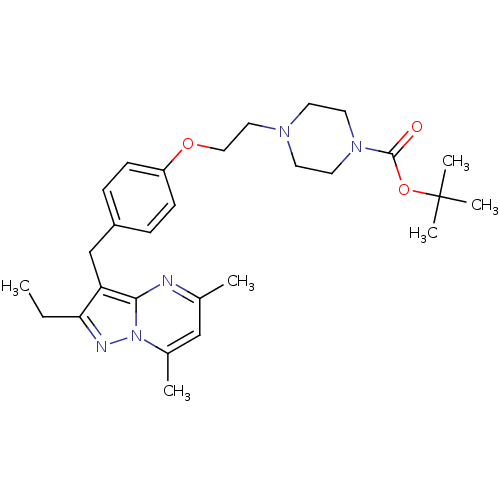 (US8748435, 26) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 44 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123478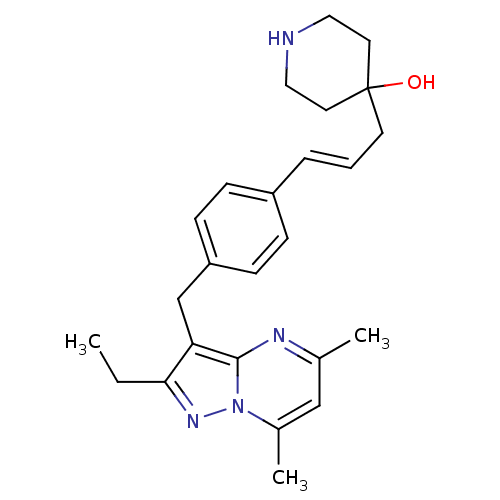 (US8748435, 3) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 44 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123476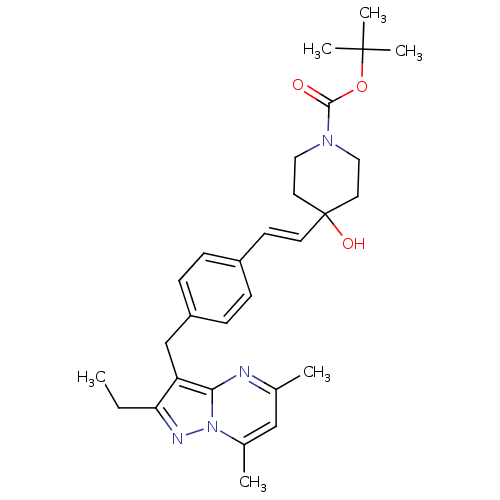 (US8748435, 1) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123479 (US8748435, 4) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 47 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM50260329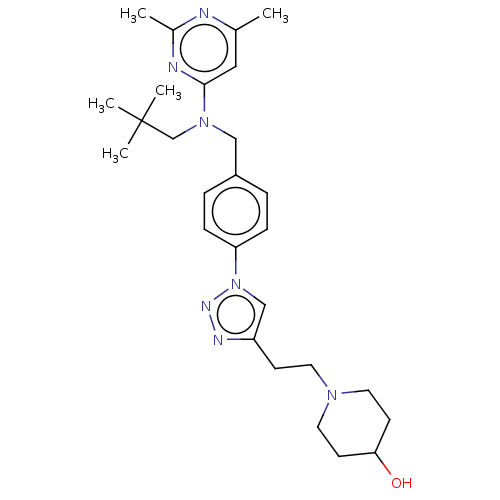 (CHEMBL4098400) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human GPR4 expressed in African green monkey COS7 cells assessed as inhibition of pH 6.5-induced cAMP accumulation after 30 mi... | Bioorg Med Chem 25: 4512-4525 (2017) Article DOI: 10.1016/j.bmc.2017.06.050 BindingDB Entry DOI: 10.7270/Q2NS0XB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM50450951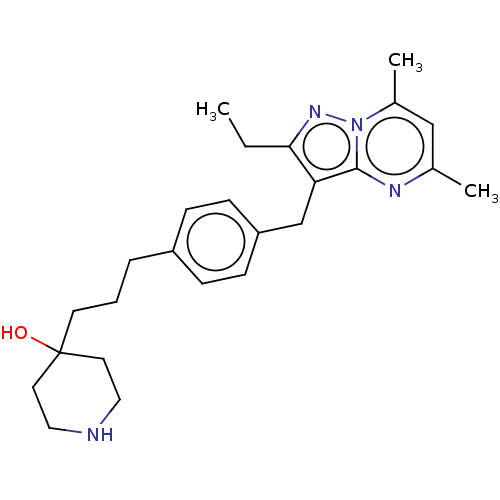 (CHEMBL4213196) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human GPR4 expressed in human HeLa cells assessed as inhibition of IBMX-induced cAMP accumulation after 15 mins by HTRF assay | J Med Chem 60: 3672-3683 (2017) Article DOI: 10.1021/acs.jmedchem.6b01703 BindingDB Entry DOI: 10.7270/Q28P6349 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123490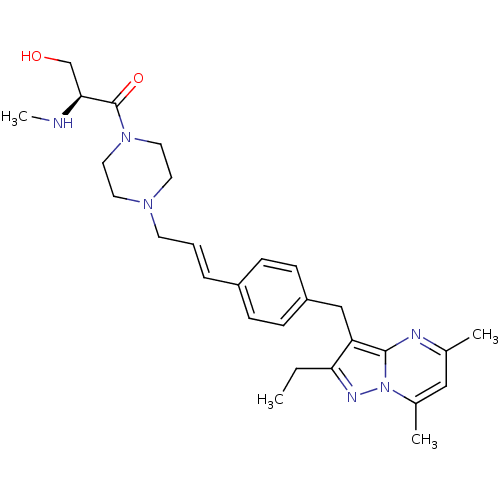 (US8748435, 15) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 51 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM50450949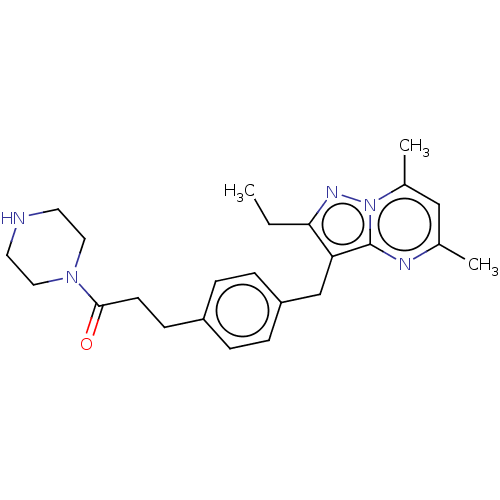 (CHEMBL4212656) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human GPR4 expressed in human HeLa cells assessed as inhibition of IBMX-induced cAMP accumulation after 15 mins by HTRF assay | J Med Chem 60: 3672-3683 (2017) Article DOI: 10.1021/acs.jmedchem.6b01703 BindingDB Entry DOI: 10.7270/Q28P6349 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123499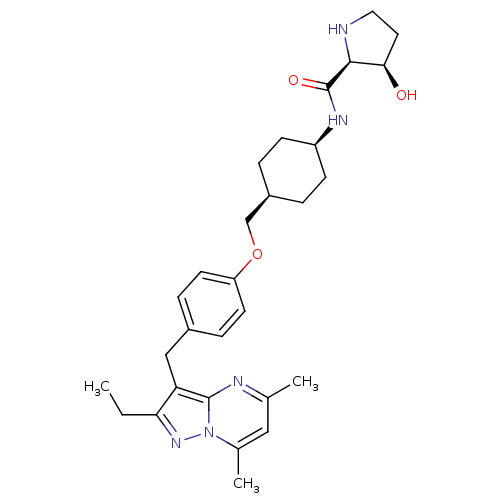 (US8748435, 24) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 54 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123494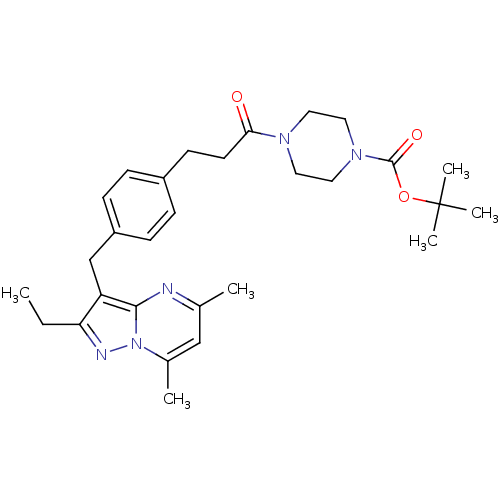 (US8748435, 19) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 54 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123515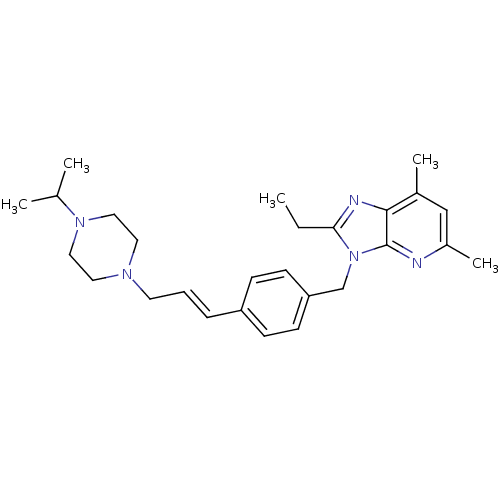 (US8748435, 40) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123502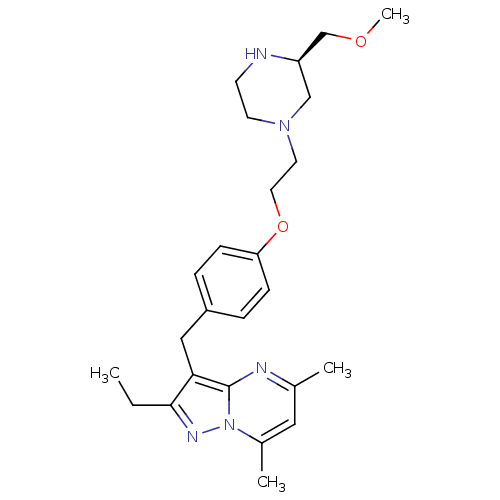 (US8748435, 27) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 59 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM50450952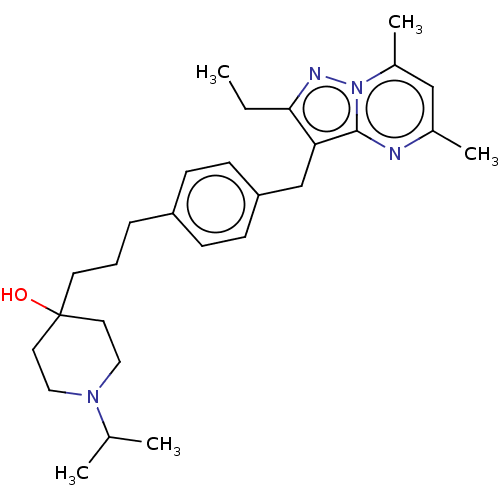 (CHEMBL4211343) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human GPR4 expressed in human HeLa cells assessed as inhibition of IBMX-induced cAMP accumulation after 15 mins by HTRF assay | J Med Chem 60: 3672-3683 (2017) Article DOI: 10.1021/acs.jmedchem.6b01703 BindingDB Entry DOI: 10.7270/Q28P6349 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM50450950 (CHEMBL4217603) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human GPR4 expressed in human HeLa cells assessed as inhibition of IBMX-induced cAMP accumulation after 15 mins in presence of... | J Med Chem 60: 3672-3683 (2017) Article DOI: 10.1021/acs.jmedchem.6b01703 BindingDB Entry DOI: 10.7270/Q28P6349 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM50175290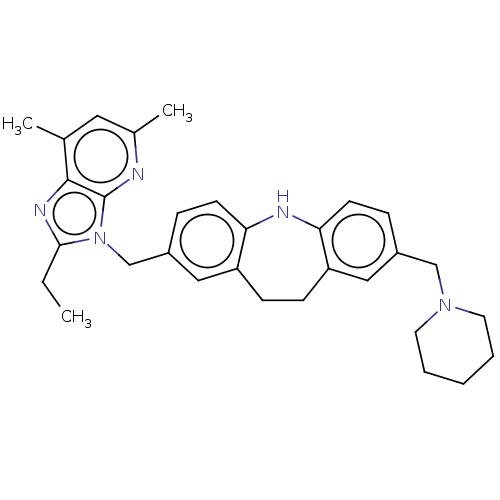 (CHEMBL3810385) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Antagonist activity at N-terminal HA-tagged GPR4 (unknown origin) expressed in HEK293 cells assessed as inhibition of pH dependent cAMP response elem... | ACS Med Chem Lett 7: 493-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00014 BindingDB Entry DOI: 10.7270/Q2833TZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123510 (US8748435, 35) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human GPR4 expressed in human HeLa cells assessed as inhibition of IBMX-induced cAMP accumulation after 15 mins in presence of... | J Med Chem 60: 3672-3683 (2017) Article DOI: 10.1021/acs.jmedchem.6b01703 BindingDB Entry DOI: 10.7270/Q28P6349 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123510 (US8748435, 35) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human GPR4 expressed in human HeLa cells assessed as inhibition of IBMX-induced cAMP accumulation after 15 mins by HTRF assay | J Med Chem 60: 3672-3683 (2017) Article DOI: 10.1021/acs.jmedchem.6b01703 BindingDB Entry DOI: 10.7270/Q28P6349 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM50450948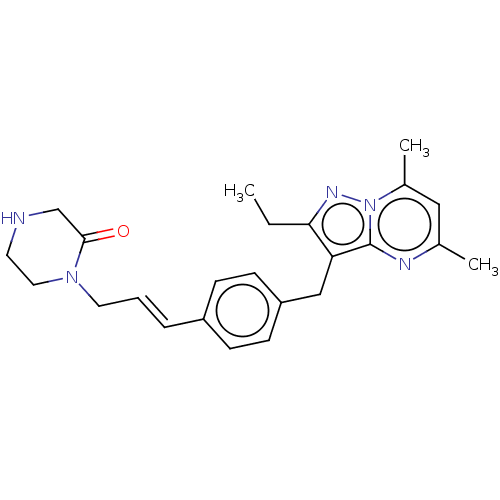 (CHEMBL4207309) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human GPR4 expressed in human HeLa cells assessed as inhibition of IBMX-induced cAMP accumulation after 15 mins by HTRF assay | J Med Chem 60: 3672-3683 (2017) Article DOI: 10.1021/acs.jmedchem.6b01703 BindingDB Entry DOI: 10.7270/Q28P6349 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123510 (US8748435, 35) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 74 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM50450955 (CHEMBL4205308) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human GPR4 expressed in human HeLa cells assessed as inhibition of IBMX-induced cAMP accumulation after 15 mins by HTRF assay | J Med Chem 60: 3672-3683 (2017) Article DOI: 10.1021/acs.jmedchem.6b01703 BindingDB Entry DOI: 10.7270/Q28P6349 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123509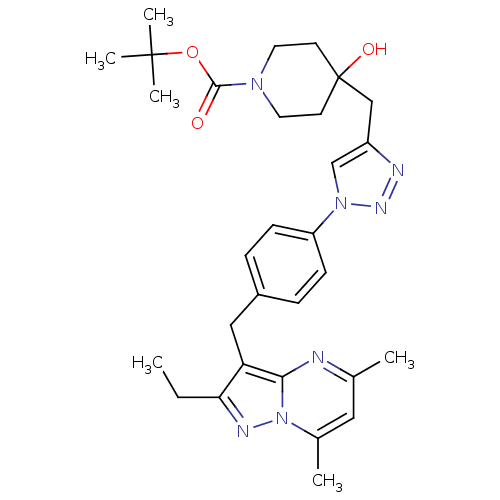 (US8748435, 34) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 77 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123493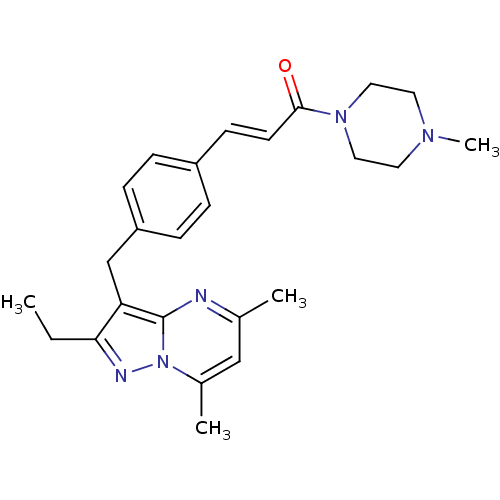 (US8748435, 18) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 81 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 84 total ) | Next | Last >> |