 Found 585 hits of ki for UniProtKB: P43115
Found 585 hits of ki for UniProtKB: P43115 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM85177
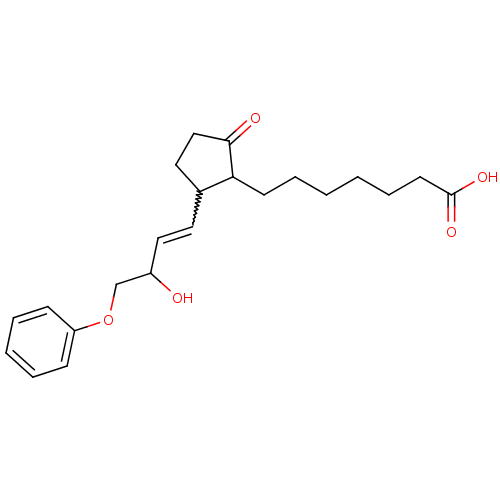
(CAS_80558-61-8 | M&B-28767 | NSC_119139)Show SMILES OC(COc1ccccc1)C=CC1CCC(=O)C1CCCCCCC(O)=O |w:11.12| Show InChI InChI=1S/C22H30O5/c23-18(16-27-19-8-4-3-5-9-19)14-12-17-13-15-21(24)20(17)10-6-1-2-7-11-22(25)26/h3-5,8-9,12,14,17-18,20,23H,1-2,6-7,10-11,13,15-16H2,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 4323-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.025
BindingDB Entry DOI: 10.7270/Q2RF5VVC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM85183
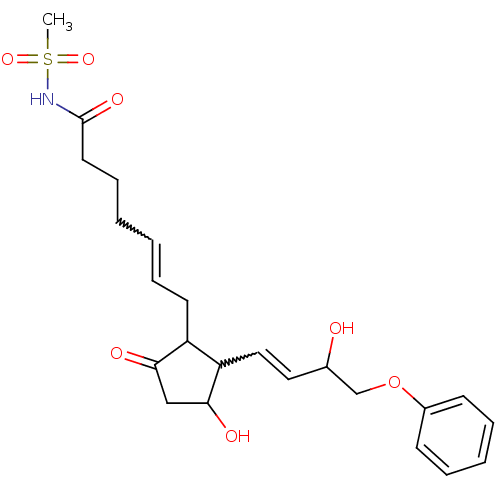
(CAS_60325-46-4 | NSC_43251 | SULPROSTONE)Show SMILES CS(=O)(=O)NC(=O)CCCC=CCC1C(C=CC(O)COc2ccccc2)C(O)CC1=O |w:10.9,15.14| Show InChI InChI=1S/C23H31NO7S/c1-32(29,30)24-23(28)12-8-3-2-7-11-19-20(22(27)15-21(19)26)14-13-17(25)16-31-18-9-5-4-6-10-18/h2,4-7,9-10,13-14,17,19-20,22,25,27H,3,8,11-12,15-16H2,1H3,(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50580024

(CHEMBL5092858)Show SMILES FC(F)(F)c1ccc(cc1)-n1ncc2CCC\C(=C/CNC(=O)NS(=O)(=O)c3cc(Cl)c(Cl)s3)c12 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-PGE2 from human EP3 receptor assessed as inhibition constant incubated for 2 hrs by TopCount scintillation counting method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00594
BindingDB Entry DOI: 10.7270/Q2HT2T6X |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50580013

(CHEMBL5086191)Show SMILES Clc1cc(sc1Cl)S(=O)(=O)NC(=O)NC\C=C1/CCCc2cnn(Cc3ccc(Cl)cc3Cl)c12 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-PGE2 from human EP3 receptor assessed as inhibition constant incubated for 2 hrs by TopCount scintillation counting method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00594
BindingDB Entry DOI: 10.7270/Q2HT2T6X |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193922
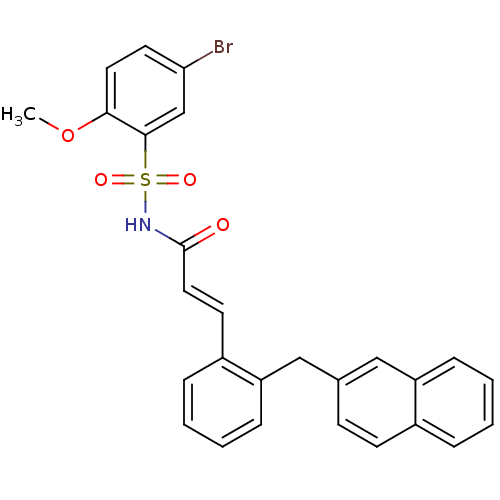
(CHEMBL218071 | N-(5-bromo-2-methoxyphenylsulfonyl)...)Show SMILES COc1ccc(Br)cc1S(=O)(=O)NC(=O)\C=C\c1ccccc1Cc1ccc2ccccc2c1 Show InChI InChI=1S/C27H22BrNO4S/c1-33-25-14-13-24(28)18-26(25)34(31,32)29-27(30)15-12-21-7-3-5-9-23(21)17-19-10-11-20-6-2-4-8-22(20)16-19/h2-16,18H,17H2,1H3,(H,29,30)/b15-12+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193920
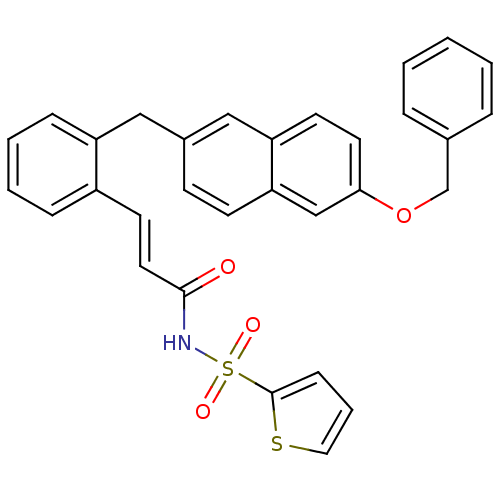
(3-(2-((6-(benzyloxy)naphthalen-2-yl)methyl)phenyl)...)Show SMILES O=C(NS(=O)(=O)c1cccs1)\C=C\c1ccccc1Cc1ccc2cc(OCc3ccccc3)ccc2c1 Show InChI InChI=1S/C31H25NO4S2/c33-30(32-38(34,35)31-11-6-18-37-31)17-15-25-9-4-5-10-26(25)19-24-12-13-28-21-29(16-14-27(28)20-24)36-22-23-7-2-1-3-8-23/h1-18,20-21H,19,22H2,(H,32,33)/b17-15+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193935

(3-(2-((6-(benzyloxy)naphthalen-2-yl)methyl)phenyl)...)Show SMILES COc1ccc(Br)cc1S(=O)(=O)NC(=O)\C=C\c1ccccc1Cc1ccc2cc(OCc3ccccc3)ccc2c1 Show InChI InChI=1S/C34H28BrNO5S/c1-40-32-17-15-30(35)22-33(32)42(38,39)36-34(37)18-14-26-9-5-6-10-27(26)19-25-11-12-29-21-31(16-13-28(29)20-25)41-23-24-7-3-2-4-8-24/h2-18,20-22H,19,23H2,1H3,(H,36,37)/b18-14+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor in presence of HSA |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193935

(3-(2-((6-(benzyloxy)naphthalen-2-yl)methyl)phenyl)...)Show SMILES COc1ccc(Br)cc1S(=O)(=O)NC(=O)\C=C\c1ccccc1Cc1ccc2cc(OCc3ccccc3)ccc2c1 Show InChI InChI=1S/C34H28BrNO5S/c1-40-32-17-15-30(35)22-33(32)42(38,39)36-34(37)18-14-26-9-5-6-10-27(26)19-25-11-12-29-21-31(16-13-28(29)20-25)41-23-24-7-3-2-4-8-24/h2-18,20-22H,19,23H2,1H3,(H,36,37)/b18-14+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50580032

(CHEMBL5078847)Show SMILES F\C(CNC(=O)NS(=O)(=O)c1cc(Cl)c(Cl)s1)=C1\CCCc2cnn(Cc3ccc(Cl)cc3Cl)c12 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-PGE2 from human EP3 receptor assessed as inhibition constant incubated for 2 hrs by TopCount scintillation counting method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00594
BindingDB Entry DOI: 10.7270/Q2HT2T6X |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50571262
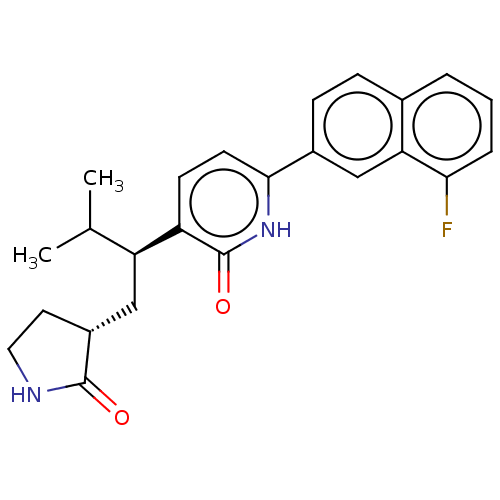
(CHEMBL4858203)Show SMILES [H][C@]1(C[C@@H](C(C)C)c2ccc([nH]c2=O)-c2ccc3cccc(F)c3c2)CCNC1=O |r| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor expressed in CHO cells by radioligand competition binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128172
BindingDB Entry DOI: 10.7270/Q2057KQF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50580015

(CHEMBL5094127)Show SMILES Clc1cc(sc1Cl)S(=O)(=O)NC(=O)OC\C=C1/CCCc2cnn(Cc3ccc(Cl)cc3Cl)c12 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-PGE2 from human EP3 receptor assessed as inhibition constant incubated for 2 hrs by TopCount scintillation counting method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00594
BindingDB Entry DOI: 10.7270/Q2HT2T6X |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50580025
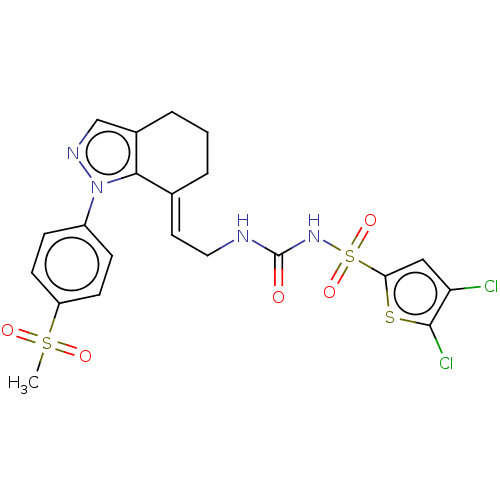
(CHEMBL5080528)Show SMILES CS(=O)(=O)c1ccc(cc1)-n1ncc2CCC\C(=C/CNC(=O)NS(=O)(=O)c3cc(Cl)c(Cl)s3)c12 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-PGE2 from human EP3 receptor assessed as inhibition constant incubated for 2 hrs by TopCount scintillation counting method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00594
BindingDB Entry DOI: 10.7270/Q2HT2T6X |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50580027

(CHEMBL5093140)Show SMILES Fc1ccc(cc1)N1CCC(CC1)n1ncc2CCC\C(=C/CNC(=O)NS(=O)(=O)c3cc(Cl)c(Cl)s3)c12 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-PGE2 from human EP3 receptor assessed as inhibition constant incubated for 2 hrs by TopCount scintillation counting method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00594
BindingDB Entry DOI: 10.7270/Q2HT2T6X |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50571236

(CHEMBL4871125)Show SMILES CC(C)NC(=O)C(C(C)C)c1ccc([nH]c1=O)-c1ccc2cccc(F)c2c1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor expressed in CHO cells by radioligand competition binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128172
BindingDB Entry DOI: 10.7270/Q2057KQF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193921
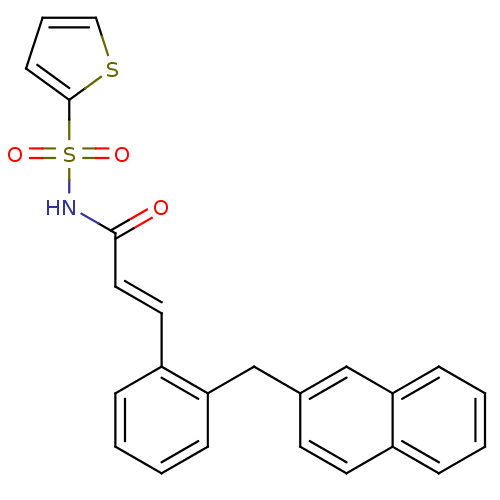
(3-(2-(naphthalen-2-ylmethyl)phenyl)-N-(thiophen-2-...)Show SMILES O=C(NS(=O)(=O)c1cccs1)\C=C\c1ccccc1Cc1ccc2ccccc2c1 Show InChI InChI=1S/C24H19NO3S2/c26-23(25-30(27,28)24-10-5-15-29-24)14-13-20-7-2-4-9-22(20)17-18-11-12-19-6-1-3-8-21(19)16-18/h1-16H,17H2,(H,25,26)/b14-13+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of EP3 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414547

(CHEMBL558644)Show SMILES CN1CCOc2ccc(cc12)C(=O)Nc1nnc(s1)-c1c(Cl)cccc1Cl Show InChI InChI=1S/C18H14Cl2N4O2S/c1-24-7-8-26-14-6-5-10(9-13(14)24)16(25)21-18-23-22-17(27-18)15-11(19)3-2-4-12(15)20/h2-6,9H,7-8H2,1H3,(H,21,23,25) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414549
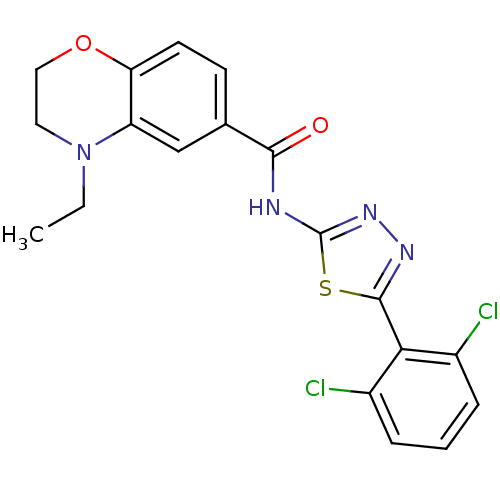
(CHEMBL563480)Show SMILES CCN1CCOc2ccc(cc12)C(=O)Nc1nnc(s1)-c1c(Cl)cccc1Cl Show InChI InChI=1S/C19H16Cl2N4O2S/c1-2-25-8-9-27-15-7-6-11(10-14(15)25)17(26)22-19-24-23-18(28-19)16-12(20)4-3-5-13(16)21/h3-7,10H,2,8-9H2,1H3,(H,22,24,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193921

(3-(2-(naphthalen-2-ylmethyl)phenyl)-N-(thiophen-2-...)Show SMILES O=C(NS(=O)(=O)c1cccs1)\C=C\c1ccccc1Cc1ccc2ccccc2c1 Show InChI InChI=1S/C24H19NO3S2/c26-23(25-30(27,28)24-10-5-15-29-24)14-13-20-7-2-4-9-22(20)17-18-11-12-19-6-1-3-8-21(19)16-18/h1-16H,17H2,(H,25,26)/b14-13+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193938

(3-(2-(4-(benzyloxy)-3-methoxycinnamyl)phenyl)-N-(t...)Show SMILES COc1cc(\C=C\Cc2ccccc2\C=C\C(=O)NS(=O)(=O)c2cccs2)ccc1OCc1ccccc1 Show InChI InChI=1S/C30H27NO5S2/c1-35-28-21-23(16-18-27(28)36-22-24-9-3-2-4-10-24)11-7-14-25-12-5-6-13-26(25)17-19-29(32)31-38(33,34)30-15-8-20-37-30/h2-13,15-21H,14,22H2,1H3,(H,31,32)/b11-7+,19-17+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193918
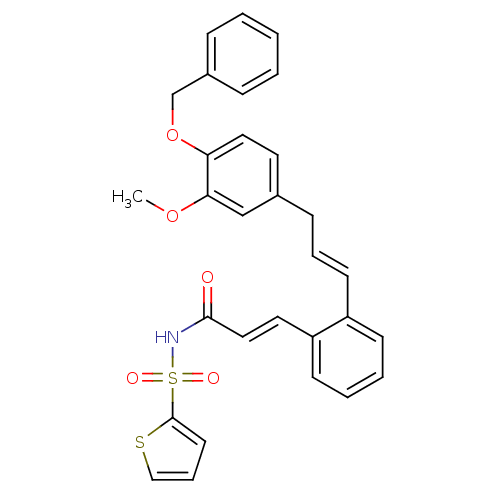
(3-(2-((E)-3-(4-(benzyloxy)-3-methoxyphenyl)prop-1-...)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(=O)NS(=O)(=O)c2cccs2)ccc1OCc1ccccc1 Show InChI InChI=1S/C30H27NO5S2/c1-35-28-21-23(16-18-27(28)36-22-24-9-3-2-4-10-24)11-7-14-25-12-5-6-13-26(25)17-19-29(32)31-38(33,34)30-15-8-20-37-30/h2-10,12-21H,11,22H2,1H3,(H,31,32)/b14-7+,19-17+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50580033
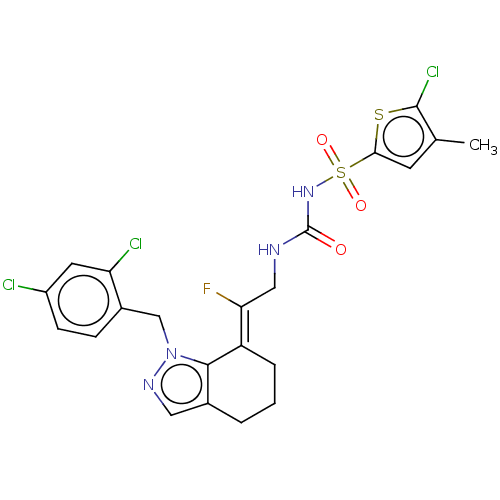
(CHEMBL5084190)Show SMILES Cc1cc(sc1Cl)S(=O)(=O)NC(=O)NC\C(F)=C1/CCCc2cnn(Cc3ccc(Cl)cc3Cl)c12 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-PGE2 from human EP3 receptor assessed as inhibition constant incubated for 2 hrs by TopCount scintillation counting method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00594
BindingDB Entry DOI: 10.7270/Q2HT2T6X |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50580021
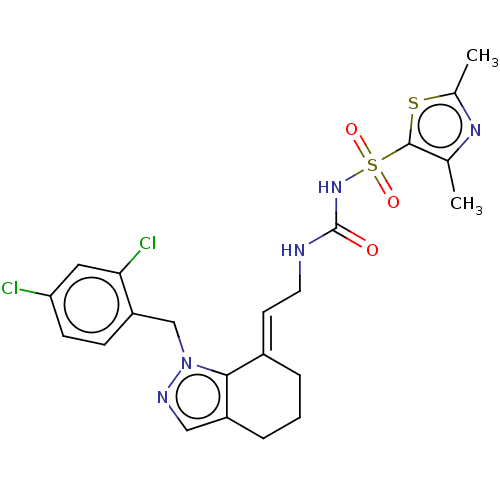
(CHEMBL5080602)Show SMILES Cc1nc(C)c(s1)S(=O)(=O)NC(=O)NC\C=C1/CCCc2cnn(Cc3ccc(Cl)cc3Cl)c12 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-PGE2 from human EP3 receptor assessed as inhibition constant incubated for 2 hrs by TopCount scintillation counting method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00594
BindingDB Entry DOI: 10.7270/Q2HT2T6X |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50095296
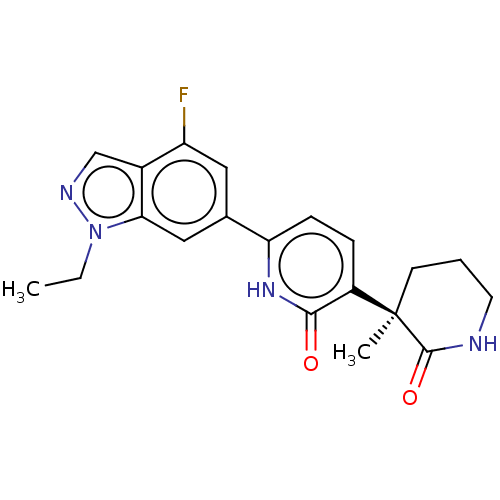
(CHEMBL3589082 | US9278953, 1)Show SMILES CCn1ncc2c(F)cc(cc12)-c1ccc(c(=O)[nH]1)[C@@]1(C)CCCNC1=O |r| Show InChI InChI=1S/C20H21Cl2N5O/c1-26-5-7-27(8-6-26)20-15-9-13(28-2)3-4-14(15)19(24-25-20)10-16-17(21)11-23-12-18(16)22/h3-4,9,11-12H,5-8,10H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| 2 | -11.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ... |
US Patent US9278953 (2016)
BindingDB Entry DOI: 10.7270/Q2W37V5Z |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50580026

(CHEMBL5091517)Show SMILES COc1ccc(cn1)-n1ncc2CCC\C(=C/CNC(=O)NS(=O)(=O)c3cc(Cl)c(Cl)s3)c12 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-PGE2 from human EP3 receptor assessed as inhibition constant incubated for 2 hrs by TopCount scintillation counting method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00594
BindingDB Entry DOI: 10.7270/Q2HT2T6X |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50095296

(CHEMBL3589082 | US9278953, 1)Show SMILES CCn1ncc2c(F)cc(cc12)-c1ccc(c(=O)[nH]1)[C@@]1(C)CCCNC1=O |r| Show InChI InChI=1S/C20H21Cl2N5O/c1-26-5-7-27(8-6-26)20-15-9-13(28-2)3-4-14(15)19(24-25-20)10-16-17(21)11-23-12-18(16)22/h3-4,9,11-12H,5-8,10H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor by MicroBeta plate-based scintillation counting/SPA binding assay |
ACS Med Chem Lett 6: 626-7 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00188
BindingDB Entry DOI: 10.7270/Q2GX4D9S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50571247
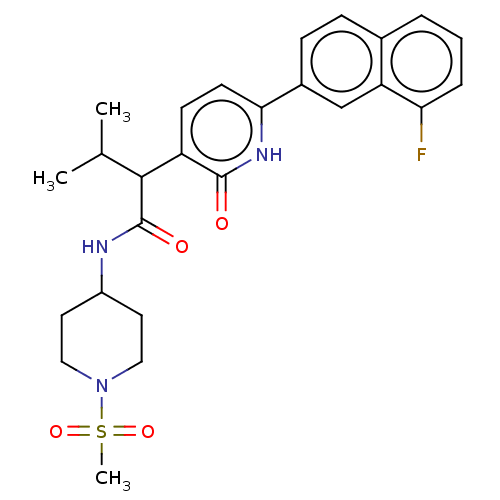
(CHEMBL4866485)Show SMILES CC(C)C(C(=O)NC1CCN(CC1)S(C)(=O)=O)c1ccc([nH]c1=O)-c1ccc2cccc(F)c2c1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor expressed in CHO cells by radioligand competition binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128172
BindingDB Entry DOI: 10.7270/Q2057KQF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50400818

(CHEMBL2204339)Show SMILES O=C(NS(=O)(=O)c1cccs1)\C=C\c1ccccc1S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C23H17NO5S3/c25-22(24-32(28,29)23-10-5-15-30-23)14-12-18-7-3-4-9-21(18)31(26,27)20-13-11-17-6-1-2-8-19(17)16-20/h1-16H,(H,24,25)/b14-12+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of EP3 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... |
J Med Chem 62: 4731-4741 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00336
BindingDB Entry DOI: 10.7270/Q22R3W32 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50571259
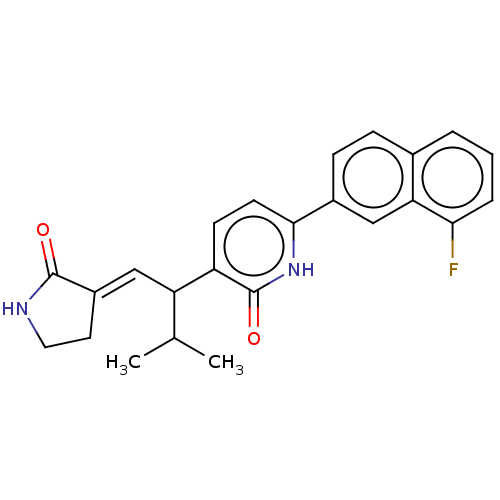
(CHEMBL4849504)Show SMILES CC(C)C(\C=C1/CCNC1=O)c1ccc([nH]c1=O)-c1ccc2cccc(F)c2c1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor expressed in CHO cells by radioligand competition binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128172
BindingDB Entry DOI: 10.7270/Q2057KQF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193923
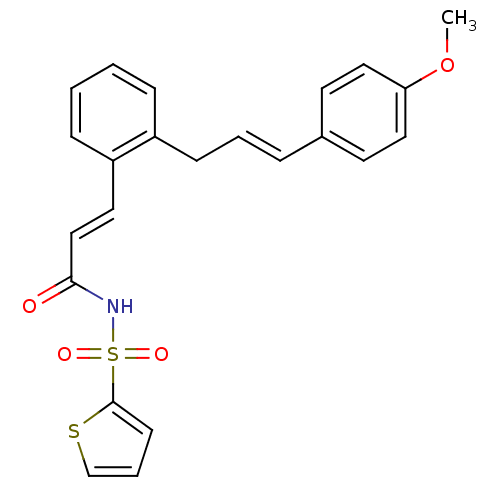
(3-(2-(4-methoxycinnamyl)phenyl)-N-(thiophen-2-ylsu...)Show SMILES COc1ccc(\C=C\Cc2ccccc2\C=C\C(=O)NS(=O)(=O)c2cccs2)cc1 Show InChI InChI=1S/C23H21NO4S2/c1-28-21-14-11-18(12-15-21)6-4-9-19-7-2-3-8-20(19)13-16-22(25)24-30(26,27)23-10-5-17-29-23/h2-8,10-17H,9H2,1H3,(H,24,25)/b6-4+,16-13+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193937

(3-(2-((E)-3-(4-methoxyphenyl)prop-1-enyl)phenyl)-N...)Show SMILES COc1ccc(C\C=C\c2ccccc2\C=C\C(=O)NS(=O)(=O)c2cccs2)cc1 Show InChI InChI=1S/C23H21NO4S2/c1-28-21-14-11-18(12-15-21)6-4-9-19-7-2-3-8-20(19)13-16-22(25)24-30(26,27)23-10-5-17-29-23/h2-5,7-17H,6H2,1H3,(H,24,25)/b9-4+,16-13+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50571244
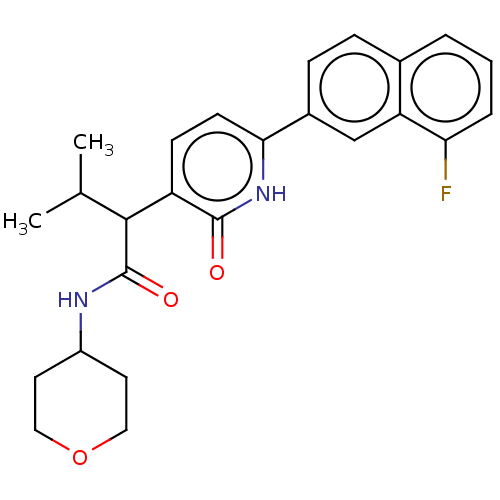
(CHEMBL4846309)Show SMILES CC(C)C(C(=O)NC1CCOCC1)c1ccc([nH]c1=O)-c1ccc2cccc(F)c2c1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor expressed in CHO cells by radioligand competition binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128172
BindingDB Entry DOI: 10.7270/Q2057KQF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193920

(3-(2-((6-(benzyloxy)naphthalen-2-yl)methyl)phenyl)...)Show SMILES O=C(NS(=O)(=O)c1cccs1)\C=C\c1ccccc1Cc1ccc2cc(OCc3ccccc3)ccc2c1 Show InChI InChI=1S/C31H25NO4S2/c33-30(32-38(34,35)31-11-6-18-37-31)17-15-25-9-4-5-10-26(25)19-24-12-13-28-21-29(16-14-27(28)20-24)36-22-23-7-2-1-3-8-23/h1-18,20-21H,19,22H2,(H,32,33)/b17-15+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor in presence of HSA |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50571253
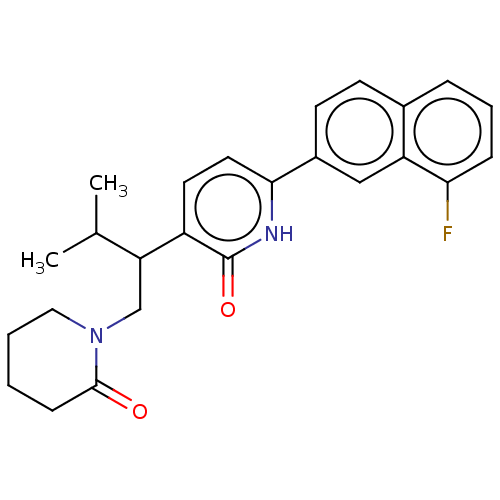
(CHEMBL4867987)Show SMILES CC(C)C(CN1CCCCC1=O)c1ccc([nH]c1=O)-c1ccc2cccc(F)c2c1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor expressed in CHO cells by radioligand competition binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128172
BindingDB Entry DOI: 10.7270/Q2057KQF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50571245

(CHEMBL4875575)Show SMILES CC(C)C(C(=O)NC1CCS(=O)(=O)CC1)c1ccc([nH]c1=O)-c1ccc2cccc(F)c2c1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor expressed in CHO cells by radioligand competition binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128172
BindingDB Entry DOI: 10.7270/Q2057KQF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50571238

(CHEMBL4856048)Show SMILES CONC(=O)C(C(C)C)c1ccc([nH]c1=O)-c1ccc2cccc(F)c2c1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor expressed in CHO cells by radioligand competition binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128172
BindingDB Entry DOI: 10.7270/Q2057KQF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50580031

(CHEMBL5083128)Show SMILES COc1ccc(cc1Cl)S(=O)(=O)NC(=O)NC\C(F)=C1/CCCc2cnn(Cc3ccc(Cl)cc3Cl)c12 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-PGE2 from human EP3 receptor assessed as inhibition constant incubated for 2 hrs by TopCount scintillation counting method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00594
BindingDB Entry DOI: 10.7270/Q2HT2T6X |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50580037
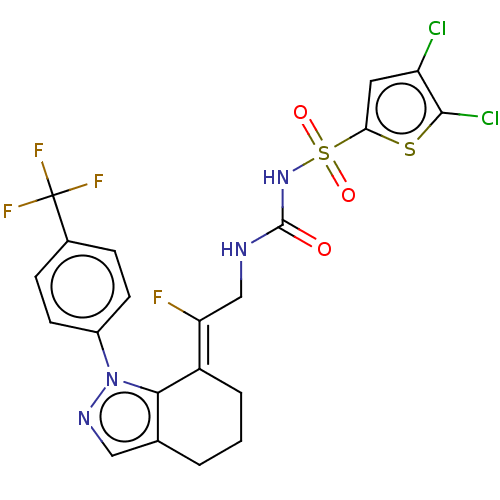
(CHEMBL5072303)Show SMILES F\C(CNC(=O)NS(=O)(=O)c1cc(Cl)c(Cl)s1)=C1\CCCc2cnn(c12)-c1ccc(cc1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-PGE2 from human EP3 receptor assessed as inhibition constant incubated for 2 hrs by TopCount scintillation counting method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00594
BindingDB Entry DOI: 10.7270/Q2HT2T6X |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50134526
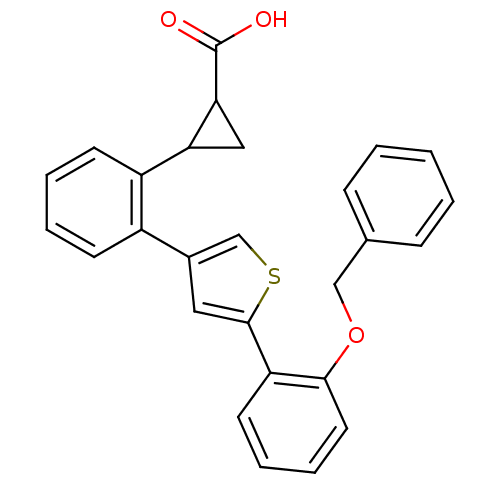
(2-{2-[5-(2-Benzyloxy-phenyl)-thiophen-3-yl]-phenyl...)Show SMILES OC(=O)C1CC1c1ccccc1-c1csc(c1)-c1ccccc1OCc1ccccc1 Show InChI InChI=1S/C27H22O3S/c28-27(29)24-15-23(24)21-11-5-4-10-20(21)19-14-26(31-17-19)22-12-6-7-13-25(22)30-16-18-8-2-1-3-9-18/h1-14,17,23-24H,15-16H2,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Affinity at human Prostanoid EP3 receptor in the human embryonic kidney (HEK) 293 cell line. |
Bioorg Med Chem Lett 13: 3813-6 (2003)
BindingDB Entry DOI: 10.7270/Q26T0M1N |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193926

(3-(2-(2-(2,6-dichlorobenzyloxy)-5-methylcinnamyl)p...)Show SMILES Cc1ccc(OCc2c(Cl)cccc2Cl)c(\C=C\Cc2ccccc2\C=C\C(O)=O)c1 Show InChI InChI=1S/C26H22Cl2O3/c1-18-12-14-25(31-17-22-23(27)10-5-11-24(22)28)21(16-18)9-4-8-19-6-2-3-7-20(19)13-15-26(29)30/h2-7,9-16H,8,17H2,1H3,(H,29,30)/b9-4+,15-13+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50159778
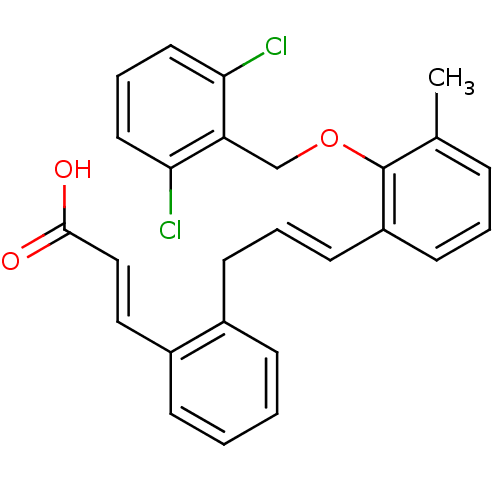
((E)-3-(2-{(E)-3-[2-(2,6-Dichloro-benzyloxy)-3-meth...)Show SMILES Cc1cccc(\C=C\Cc2ccccc2\C=C\C(O)=O)c1OCc1c(Cl)cccc1Cl Show InChI InChI=1S/C26H22Cl2O3/c1-18-7-4-11-21(26(18)31-17-22-23(27)13-6-14-24(22)28)12-5-10-19-8-2-3-9-20(19)15-16-25(29)30/h2-9,11-16H,10,17H2,1H3,(H,29,30)/b12-5+,16-15+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity for human prostanoid EP3 receptor |
Bioorg Med Chem Lett 15: 527-30 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.051
BindingDB Entry DOI: 10.7270/Q26H4J51 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50553041
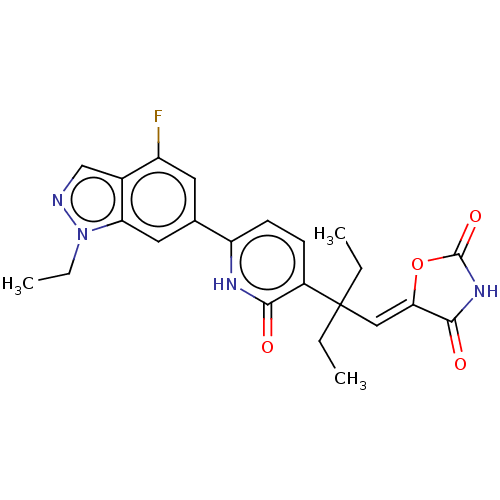
(CHEMBL4779295)Show SMILES CCn1ncc2c(F)cc(cc12)-c1ccc(c(=O)[nH]1)C(CC)(CC)\C=C1/OC(=O)NC1=O | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-PGE2 from human EP3 expressed in Chem-1 cell membranes incubated for 2 hrs by TopCount scintillation plate reader analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00667
BindingDB Entry DOI: 10.7270/Q2M61PWK |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50580018

(CHEMBL5076035)Show SMILES COc1ccc(Cl)cc1S(=O)(=O)NC(=O)NC\C=C1/CCCc2cnn(Cc3ccc(Cl)cc3Cl)c12 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-PGE2 from human EP3 receptor assessed as inhibition constant incubated for 2 hrs by TopCount scintillation counting method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00594
BindingDB Entry DOI: 10.7270/Q2HT2T6X |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384444

(CHEMBL2035510)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc2cccc(F)c2c1 Show InChI InChI=1S/C21H17FN2O4/c1-11(2)21(19(26)24-20(27)28-21)15-8-9-17(23-18(15)25)13-7-6-12-4-3-5-16(22)14(12)10-13/h3-11H,1-2H3,(H,23,25)(H,24,26,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414536
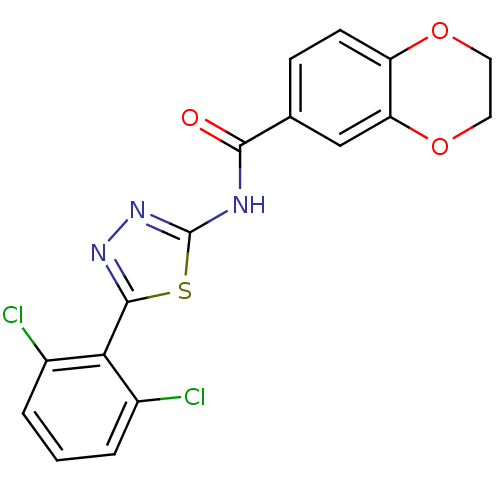
(CHEMBL551202)Show SMILES Clc1cccc(Cl)c1-c1nnc(NC(=O)c2ccc3OCCOc3c2)s1 Show InChI InChI=1S/C17H11Cl2N3O3S/c18-10-2-1-3-11(19)14(10)16-21-22-17(26-16)20-15(23)9-4-5-12-13(8-9)25-7-6-24-12/h1-5,8H,6-7H2,(H,20,22,23) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193924
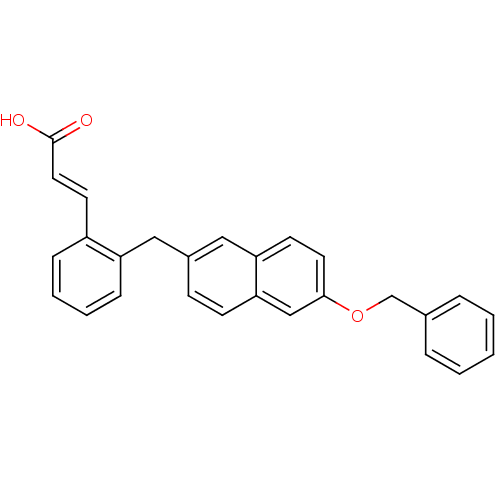
(3-(2-((6-(benzyloxy)naphthalen-2-yl)methyl)phenyl)...)Show SMILES OC(=O)\C=C\c1ccccc1Cc1ccc2cc(OCc3ccccc3)ccc2c1 Show InChI InChI=1S/C27H22O3/c28-27(29)15-13-22-8-4-5-9-23(22)16-21-10-11-25-18-26(14-12-24(25)17-21)30-19-20-6-2-1-3-7-20/h1-15,17-18H,16,19H2,(H,28,29)/b15-13+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM335698
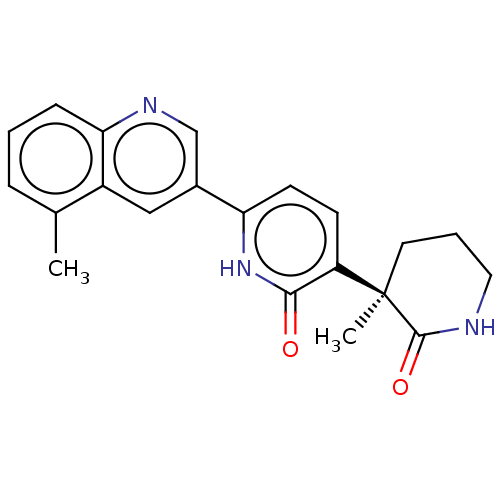
((R)-3-(3-Methyl-2-oxopiperidin-3-yl)-6-(5-methylqu...)Show SMILES Cc1cccc2ncc(cc12)-c1ccc(c(=O)[nH]1)[C@@]1(C)CCCNC1=O Show InChI InChI=1S/C21H21N3O2/c1-13-5-3-6-18-15(13)11-14(12-23-18)17-8-7-16(19(25)24-17)21(2)9-4-10-22-20(21)26/h3,5-8,11-12H,4,9-10H2,1-2H3,(H,22,26)(H,24,25)/t21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
To measure the ability of test compounds in the present invention to bind to the human EP3 receptor, and therefore have the potential to antagonize P... |
US Patent US9738626 (2017)
BindingDB Entry DOI: 10.7270/Q2CF9S7K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data