

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50297400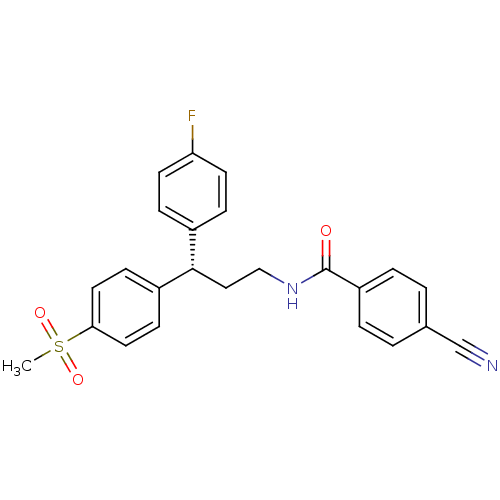 ((S)-4-Cyano-N-[3-(4-fluorophenyl)-3-(4-methanesulf...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase in human HepG cells assessed as conversion of epoxyeicosatienoic acid to dihydroxyeicosatrienoic acid a... | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50305639 (CHEMBL592741 | N-(2,4-dichlorobenzyl)-4-(3-(methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase in human HepG cells assessed as conversion of epoxyeicosatienoic acid to dihydroxyeicosatrienoic acid a... | Bioorg Med Chem Lett 20: 571-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.091 BindingDB Entry DOI: 10.7270/Q23R0SZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50305642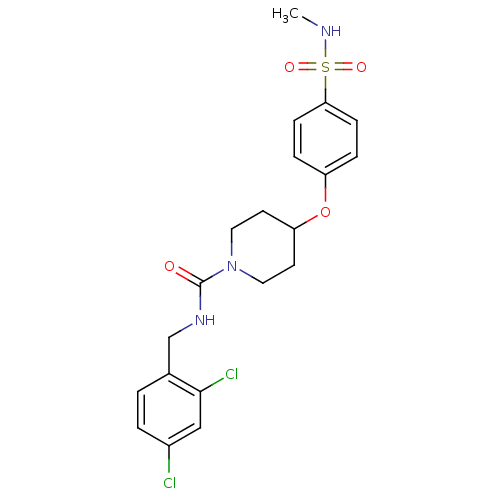 (CHEMBL589138 | N-(2,4-dichlorobenzyl)-4-(4-(N-meth...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase in human HepG cells assessed as conversion of epoxyeicosatienoic acid to dihydroxyeicosatrienoic acid a... | Bioorg Med Chem Lett 20: 571-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.091 BindingDB Entry DOI: 10.7270/Q23R0SZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50297414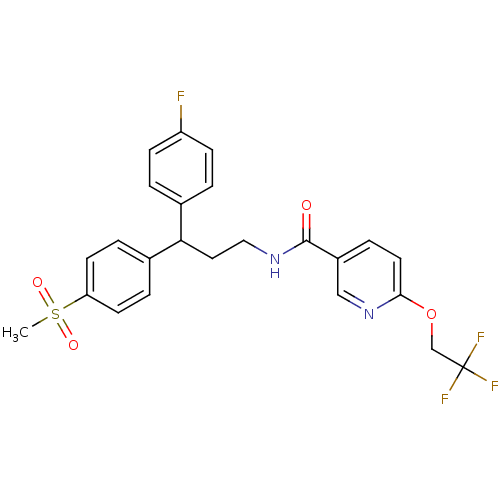 (CHEMBL556234 | N-[3-(4-Fluorophenyl)-3-(4-methanes...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase in human HepG cells assessed as conversion of epoxyeicosatienoic acid to dihydroxyeicosatrienoic acid a... | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50297400 ((S)-4-Cyano-N-[3-(4-fluorophenyl)-3-(4-methanesulf...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase in human HepG cells assessed as conversion of epoxyeicosatienoic acid to dihydroxyeicosatrienoic acid a... | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50305641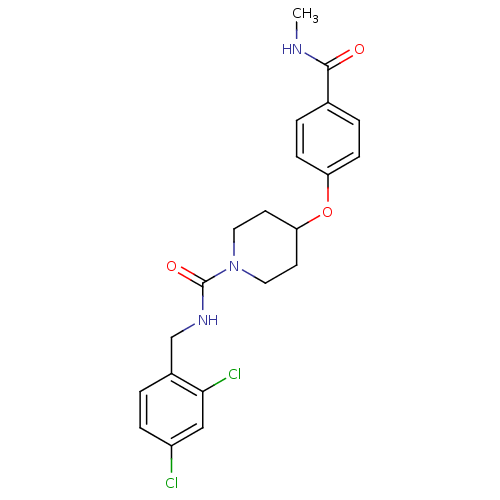 (CHEMBL592743 | N-(2,4-dichlorobenzyl)-4-(4-(methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase in human HepG cells assessed as conversion of epoxyeicosatienoic acid to dihydroxyeicosatrienoic acid a... | Bioorg Med Chem Lett 20: 571-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.091 BindingDB Entry DOI: 10.7270/Q23R0SZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50297401 (CHEMBL562081 | N-[3-(4-Fluorophenyl)-3-(4-methanes...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase in human HepG cells assessed as conversion of epoxyeicosatienoic acid to dihydroxyeicosatrienoic acid a... | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50305635 (4-(2-cyanophenoxy)-N-(2,4-dichlorobenzyl)piperidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase in human HepG cells assessed as conversion of epoxyeicosatienoic acid to dihydroxyeicosatrienoic acid a... | Bioorg Med Chem Lett 20: 571-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.091 BindingDB Entry DOI: 10.7270/Q23R0SZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50297399 (CHEMBL564145 | N-[3-(4-Fluorophenyl)-3-(4-methanes...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase in human HepG cells assessed as conversion of epoxyeicosatienoic acid to dihydroxyeicosatrienoic acid a... | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50305636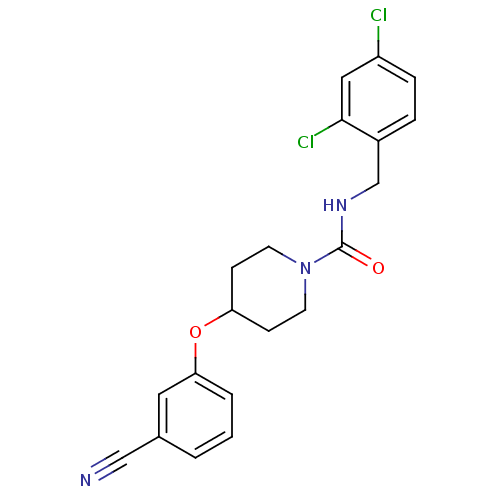 (4-(3-cyanophenoxy)-N-(2,4-dichlorobenzyl)piperidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase in human HepG cells assessed as conversion of epoxyeicosatienoic acid to dihydroxyeicosatrienoic acid a... | Bioorg Med Chem Lett 20: 571-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.091 BindingDB Entry DOI: 10.7270/Q23R0SZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50305637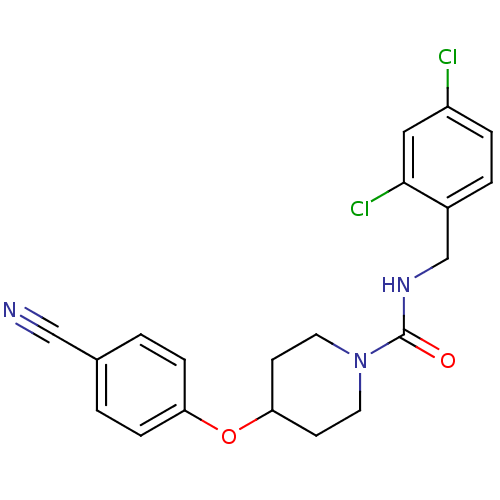 (4-(4-cyanophenoxy)-N-(2,4-dichlorobenzyl)piperidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase in human HepG cells assessed as conversion of epoxyeicosatienoic acid to dihydroxyeicosatrienoic acid a... | Bioorg Med Chem Lett 20: 571-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.091 BindingDB Entry DOI: 10.7270/Q23R0SZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50305640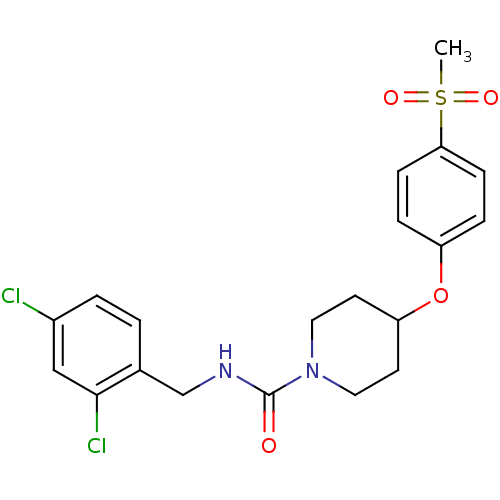 (CHEMBL592742 | N-(2,4-dichlorobenzyl)-4-(4-(methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.970 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase in human HepG cells assessed as conversion of epoxyeicosatienoic acid to dihydroxyeicosatrienoic acid a... | Bioorg Med Chem Lett 20: 571-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.091 BindingDB Entry DOI: 10.7270/Q23R0SZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50305638 (CHEMBL589860 | N-(2,4-dichlorobenzyl)-4-(2-(methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.65 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase in human HepG cells assessed as conversion of epoxyeicosatienoic acid to dihydroxyeicosatrienoic acid a... | Bioorg Med Chem Lett 20: 571-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.091 BindingDB Entry DOI: 10.7270/Q23R0SZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50414745 (CHEMBL2021549) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase in human HepG cells assessed as conversion of epoxyeicosatienoic acid to dihydroxyeicosatrienoic acid a... | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50305643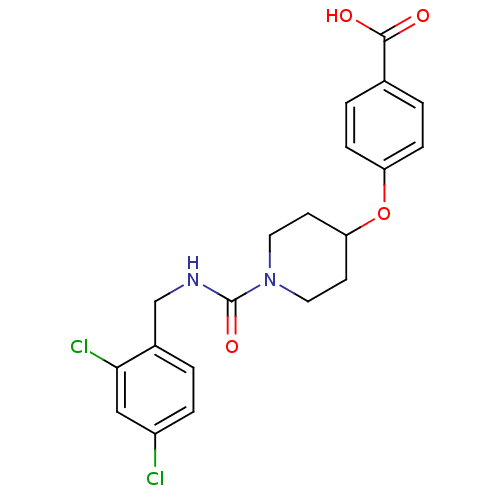 (4-(1-(2,4-dichlorobenzylcarbamoyl)piperidin-4-ylox...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase in human HepG cells assessed as conversion of epoxyeicosatienoic acid to dihydroxyeicosatrienoic acid a... | Bioorg Med Chem Lett 20: 571-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.091 BindingDB Entry DOI: 10.7270/Q23R0SZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50297395 ((S)-N-[3-(4-Fluorophenyl)-3-(4-methanesulfonyl-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase in human HepG cells assessed as conversion of epoxyeicosatienoic acid to dihydroxyeicosatrienoic acid a... | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50297403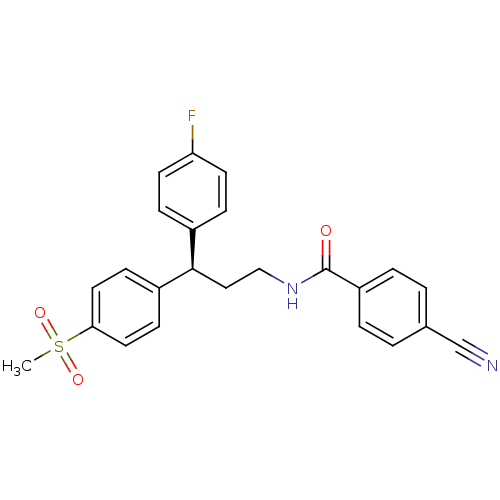 ((R)-4-Cyano-N-[3-(4-fluorophenyl)-3-(4-methanesulf...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase in human HepG cells assessed as conversion of epoxyeicosatienoic acid to dihydroxyeicosatrienoic acid a... | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50305632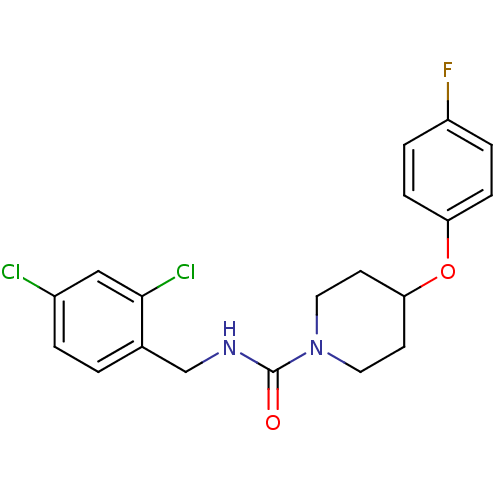 (CHEMBL590106 | N-(2,4-dichlorobenzyl)-4-(4-fluorop...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase in human HepG cells assessed as conversion of epoxyeicosatienoic acid to dihydroxyeicosatrienoic acid a... | Bioorg Med Chem Lett 20: 571-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.091 BindingDB Entry DOI: 10.7270/Q23R0SZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50305633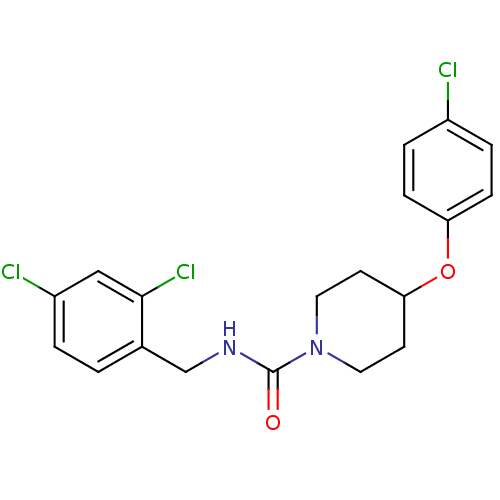 (4-(4-chlorophenoxy)-N-(2,4-dichlorobenzyl)piperidi...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase in human HepG cells assessed as conversion of epoxyeicosatienoic acid to dihydroxyeicosatrienoic acid a... | Bioorg Med Chem Lett 20: 571-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.091 BindingDB Entry DOI: 10.7270/Q23R0SZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50305634 (CHEMBL589375 | N-(2,4-dichlorobenzyl)-4-(4-(triflu...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase in human HepG cells assessed as conversion of epoxyeicosatienoic acid to dihydroxyeicosatrienoic acid a... | Bioorg Med Chem Lett 20: 571-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.091 BindingDB Entry DOI: 10.7270/Q23R0SZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50414746 (CHEMBL2021563) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase in human HepG cells assessed as conversion of epoxyeicosatienoic acid to dihydroxyeicosatrienoic acid a... | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50583627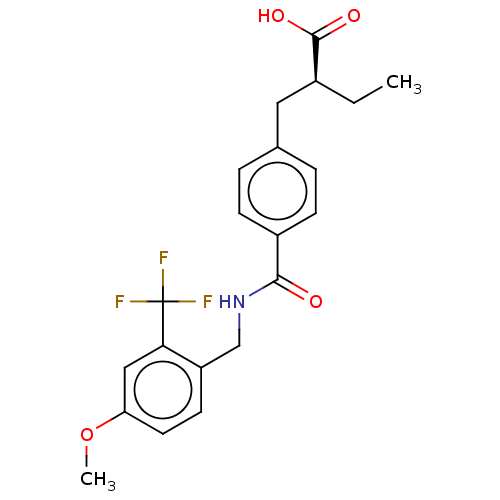 (CHEMBL5075221) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human C-terminal sEH (222 to 555 residues) expressed in Escherichia coli BL21-DE3 using PHOME as substrate assessed as redu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02063 BindingDB Entry DOI: 10.7270/Q2HM5DBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||