 Found 3138 hits of ic50 for UniProtKB: P34913
Found 3138 hits of ic50 for UniProtKB: P34913 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50264106
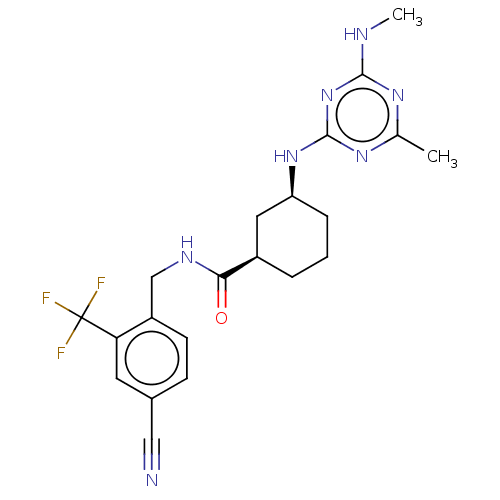
(CHEMBL3818875)Show SMILES CNc1nc(C)nc(N[C@H]2CCC[C@H](C2)C(=O)NCc2ccc(cc2C(F)(F)F)C#N)n1 |r| Show InChI InChI=1S/C21H24F3N7O/c1-12-28-19(26-2)31-20(29-12)30-16-5-3-4-14(9-16)18(32)27-11-15-7-6-13(10-25)8-17(15)21(22,23)24/h6-8,14,16H,3-5,9,11H2,1-2H3,(H,27,32)(H2,26,28,29,30,31)/t14-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sEH using MNPC as substrate by fluorescence-based assay |
J Med Chem 61: 3541-3550 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01804
BindingDB Entry DOI: 10.7270/Q2ZP48KN |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50264106

(CHEMBL3818875)Show SMILES CNc1nc(C)nc(N[C@H]2CCC[C@H](C2)C(=O)NCc2ccc(cc2C(F)(F)F)C#N)n1 |r| Show InChI InChI=1S/C21H24F3N7O/c1-12-28-19(26-2)31-20(29-12)30-16-5-3-4-14(9-16)18(32)27-11-15-7-6-13(10-25)8-17(15)21(22,23)24/h6-8,14,16H,3-5,9,11H2,1-2H3,(H,27,32)(H2,26,28,29,30,31)/t14-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation (DGMIF)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human soluble epoxide hydrolase |
J Med Chem 63: 6578-6599 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01782
BindingDB Entry DOI: 10.7270/Q2W95DQ2 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50264106

(CHEMBL3818875)Show SMILES CNc1nc(C)nc(N[C@H]2CCC[C@H](C2)C(=O)NCc2ccc(cc2C(F)(F)F)C#N)n1 |r| Show InChI InChI=1S/C21H24F3N7O/c1-12-28-19(26-2)31-20(29-12)30-16-5-3-4-14(9-16)18(32)27-11-15-7-6-13(10-25)8-17(15)21(22,23)24/h6-8,14,16H,3-5,9,11H2,1-2H3,(H,27,32)(H2,26,28,29,30,31)/t14-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sEH using 14,15-epoxy-5Z,8Z,11Z-eicosatrienoic acid as substrate assessed as formation of 14,15-dihydroxy-5Z,8Z,11Zei... |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50264106

(CHEMBL3818875)Show SMILES CNc1nc(C)nc(N[C@H]2CCC[C@H](C2)C(=O)NCc2ccc(cc2C(F)(F)F)C#N)n1 |r| Show InChI InChI=1S/C21H24F3N7O/c1-12-28-19(26-2)31-20(29-12)30-16-5-3-4-14(9-16)18(32)27-11-15-7-6-13(10-25)8-17(15)21(22,23)24/h6-8,14,16H,3-5,9,11H2,1-2H3,(H,27,32)(H2,26,28,29,30,31)/t14-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sEH using 14,15-epoxy-5Z,8Z,11Z-eicosatrienoic acid as substrate assessed as formation of 14,15-dihydroxy-5Z,8Z,11Zei... |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50595239
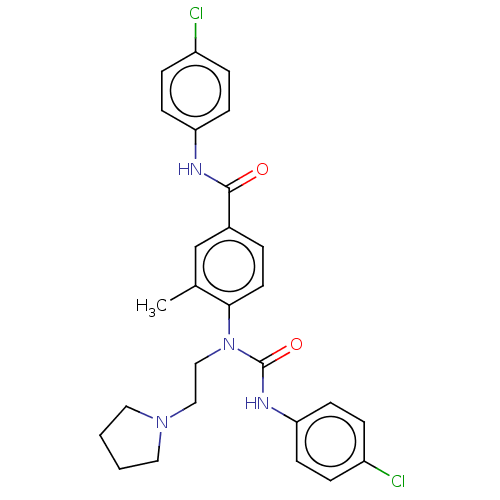
(CHEMBL5190894)Show SMILES Cc1cc(ccc1N(CCN1CCCC1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(Cl)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 3675-8 (2005)
Article DOI: 10.1016/j.bmcl.2022.128805
BindingDB Entry DOI: 10.7270/Q2J67MXV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50556773
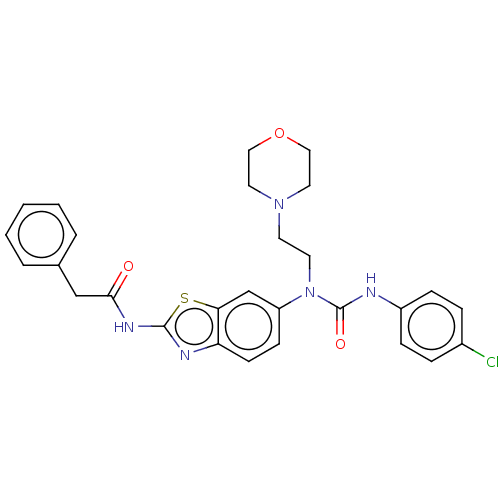
(CHEMBL4745610)Show SMILES Clc1ccc(NC(=O)N(CCN2CCOCC2)c2ccc3nc(NC(=O)Cc4ccccc4)sc3c2)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human sEH using PHOME as substrate measured after 15 mins by fluorescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113028
BindingDB Entry DOI: 10.7270/Q208690C |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50302462
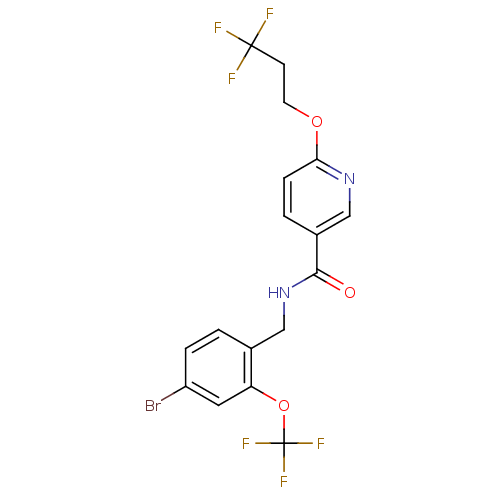
(CHEMBL566648 | N-(4-bromo-2-(trifluoromethoxy)benz...)Show SMILES FC(F)(F)CCOc1ccc(cn1)C(=O)NCc1ccc(Br)cc1OC(F)(F)F Show InChI InChI=1S/C17H13BrF6N2O3/c18-12-3-1-10(13(7-12)29-17(22,23)24)8-26-15(27)11-2-4-14(25-9-11)28-6-5-16(19,20)21/h1-4,7,9H,5-6,8H2,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of soluble EH in human HepG2 cells by cellular assay |
Bioorg Med Chem Lett 19: 5864-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.074
BindingDB Entry DOI: 10.7270/Q27944SC |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50556760

(CHEMBL4781218)Show SMILES Clc1ccc(NC(=O)Nc2nc3ccc(cc3s2)N(CCN2CCOCC2)C(=O)Nc2ccc(Cl)cc2)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human sEH using PHOME as substrate measured after 15 mins by fluorescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113028
BindingDB Entry DOI: 10.7270/Q208690C |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM342631
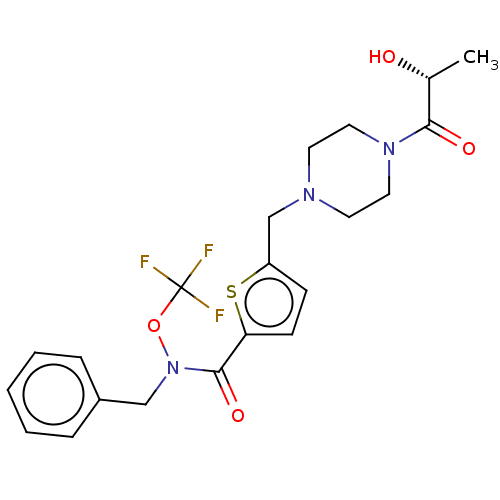
(5-[4-((R)-2-Hydroxy-propionyl)-piperazin-1-ylmethy...)Show SMILES C[C@@H](O)C(=O)N1CCN(Cc2ccc(s2)C(=O)N(Cc2ccccc2)OC(F)(F)F)CC1 Show InChI InChI=1S/C21H24F3N3O4S/c1-15(28)19(29)26-11-9-25(10-12-26)14-17-7-8-18(32-17)20(30)27(31-21(22,23)24)13-16-5-3-2-4-6-16/h2-8,15,28H,9-14H2,1H3/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.122 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI
US Patent
| Assay Description
Compounds were tested in a biochemical screening assay using recombinant sEH purified from Sf9 insect cells and an artificial substrate, (3-phenyl-ox... |
US Patent US9776991 (2017)
BindingDB Entry DOI: 10.7270/Q2V126ZH |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50445769

(CHEMBL3104615)Show SMILES Fc1cccc(F)c1C(=O)N1CCC2(CCCN(C2)C(=O)Nc2ccc(OC(F)(F)F)cc2)CC1 Show InChI InChI=1S/C24H24F5N3O3/c25-18-3-1-4-19(26)20(18)21(33)31-13-10-23(11-14-31)9-2-12-32(15-23)22(34)30-16-5-7-17(8-6-16)35-24(27,28)29/h1,3-8H,2,9-15H2,(H,30,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.125 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sEH expressed in baculovirus infected insect Sf9 cells using cyano(6-methoxynaphthalen-2-yl)methyl trans-[(3-phenylox... |
Bioorg Med Chem Lett 24: 565-70 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.020
BindingDB Entry DOI: 10.7270/Q2V69M26 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM342634

(5-[4-(Pyridine-3-carbonyl)-piperazin-1-ylmethyl]-t...)Show SMILES FC(F)(F)c1ccccc1CNC(=O)c1ccc(CN2CCN(CC2)C(=O)c2cccnc2)s1 Show InChI InChI=1S/C24H23F3N4O2S/c25-24(26,27)20-6-2-1-4-17(20)15-29-22(32)21-8-7-19(34-21)16-30-10-12-31(13-11-30)23(33)18-5-3-9-28-14-18/h1-9,14H,10-13,15-16H2,(H,29,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.168 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI
US Patent
| Assay Description
Compounds were tested in a biochemical screening assay using recombinant sEH purified from Sf9 insect cells and an artificial substrate, (3-phenyl-ox... |
US Patent US9776991 (2017)
BindingDB Entry DOI: 10.7270/Q2V126ZH |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50441467
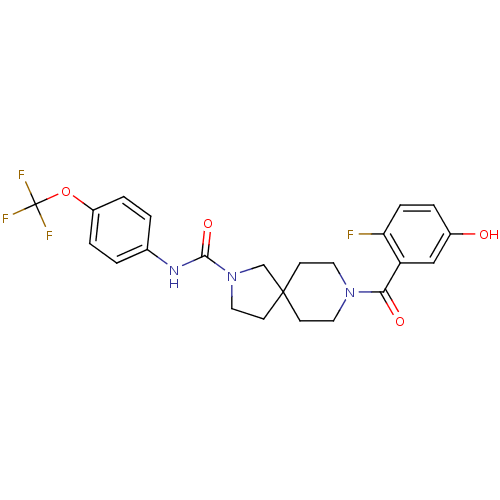
(CHEMBL2436573)Show SMILES Oc1ccc(F)c(c1)C(=O)N1CCC2(CCN(C2)C(=O)Nc2ccc(OC(F)(F)F)cc2)CC1 Show InChI InChI=1S/C23H23F4N3O4/c24-19-6-3-16(31)13-18(19)20(32)29-10-7-22(8-11-29)9-12-30(14-22)21(33)28-15-1-4-17(5-2-15)34-23(25,26)27/h1-6,13,31H,7-12,14H2,(H,28,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... |
Bioorg Med Chem Lett 23: 5975-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.054
BindingDB Entry DOI: 10.7270/Q2TT4SDR |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50441481

(CHEMBL2436579)Show SMILES FC(F)(F)Oc1ccc(NC(=O)N2CCC3(C2)CCN(CC3)C(=O)c2cnoc2C2CC2)cc1 Show InChI InChI=1S/C23H25F3N4O4/c24-23(25,26)33-17-5-3-16(4-6-17)28-21(32)30-12-9-22(14-30)7-10-29(11-8-22)20(31)18-13-27-34-19(18)15-1-2-15/h3-6,13,15H,1-2,7-12,14H2,(H,28,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... |
Bioorg Med Chem Lett 23: 5975-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.054
BindingDB Entry DOI: 10.7270/Q2TT4SDR |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50441476
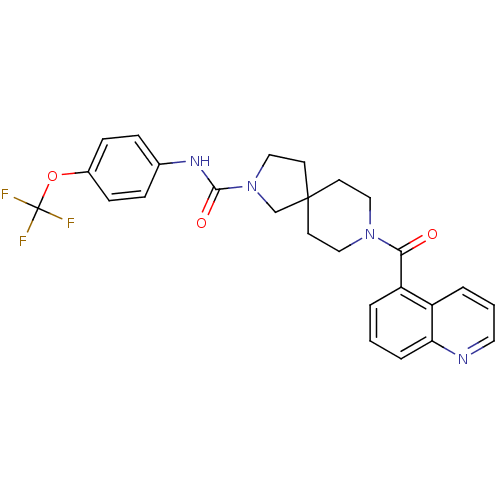
(CHEMBL2436575)Show SMILES FC(F)(F)Oc1ccc(NC(=O)N2CCC3(C2)CCN(CC3)C(=O)c2cccc3ncccc23)cc1 Show InChI InChI=1S/C26H25F3N4O3/c27-26(28,29)36-19-8-6-18(7-9-19)31-24(35)33-16-12-25(17-33)10-14-32(15-11-25)23(34)21-3-1-5-22-20(21)4-2-13-30-22/h1-9,13H,10-12,14-17H2,(H,31,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... |
Bioorg Med Chem Lett 23: 5975-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.054
BindingDB Entry DOI: 10.7270/Q2TT4SDR |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50556771
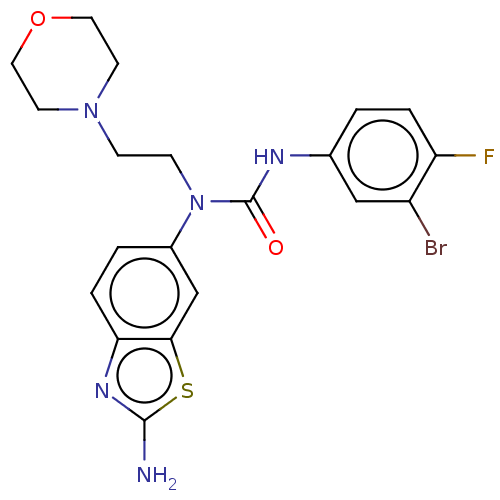
(CHEMBL4759847)Show SMILES Nc1nc2ccc(cc2s1)N(CCN1CCOCC1)C(=O)Nc1ccc(F)c(Br)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human sEH using PHOME as substrate measured after 15 mins by fluorescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113028
BindingDB Entry DOI: 10.7270/Q208690C |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50595236
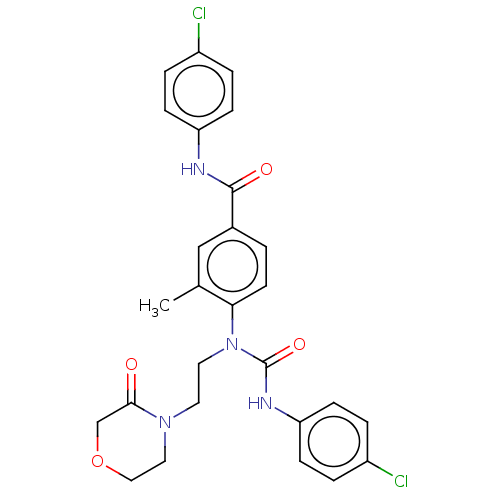
(CHEMBL5170467)Show SMILES Cc1cc(ccc1N(CCN1CCOCC1=O)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(Cl)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 3675-8 (2005)
Article DOI: 10.1016/j.bmcl.2022.128805
BindingDB Entry DOI: 10.7270/Q2J67MXV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50595244
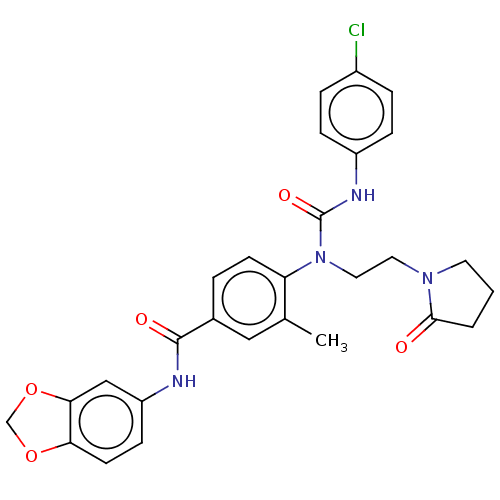
(CHEMBL5199526)Show SMILES Cc1cc(ccc1N(CCN1CCCC1=O)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc2OCOc2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 3675-8 (2005)
Article DOI: 10.1016/j.bmcl.2022.128805
BindingDB Entry DOI: 10.7270/Q2J67MXV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50441483

(CHEMBL2436591)Show SMILES CCC(CC)C(=O)N1CCC2(CCN(C2)C(=O)Nc2ccc(OC(F)(F)F)cc2)CC1 Show InChI InChI=1S/C22H30F3N3O3/c1-3-16(4-2)19(29)27-12-9-21(10-13-27)11-14-28(15-21)20(30)26-17-5-7-18(8-6-17)31-22(23,24)25/h5-8,16H,3-4,9-15H2,1-2H3,(H,26,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... |
Bioorg Med Chem Lett 23: 5975-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.054
BindingDB Entry DOI: 10.7270/Q2TT4SDR |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50441470
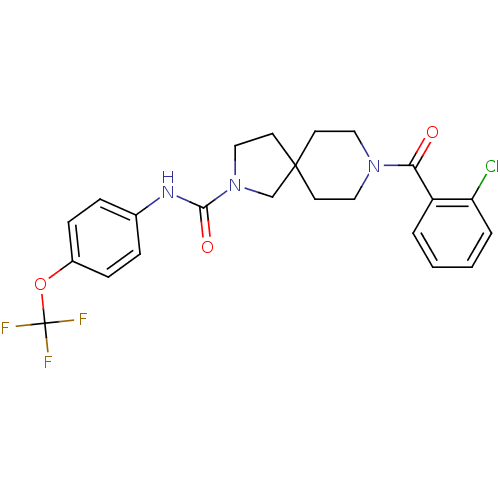
(CHEMBL2436574)Show SMILES FC(F)(F)Oc1ccc(NC(=O)N2CCC3(C2)CCN(CC3)C(=O)c2ccccc2Cl)cc1 Show InChI InChI=1S/C23H23ClF3N3O3/c24-19-4-2-1-3-18(19)20(31)29-12-9-22(10-13-29)11-14-30(15-22)21(32)28-16-5-7-17(8-6-16)33-23(25,26)27/h1-8H,9-15H2,(H,28,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... |
Bioorg Med Chem Lett 23: 5975-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.054
BindingDB Entry DOI: 10.7270/Q2TT4SDR |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50441469
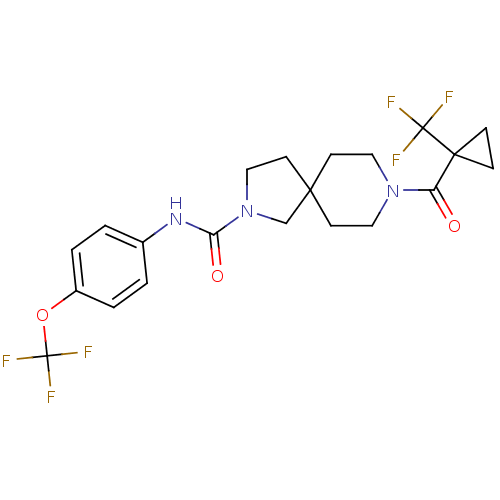
(CHEMBL2436586)Show SMILES FC(F)(F)Oc1ccc(NC(=O)N2CCC3(C2)CCN(CC3)C(=O)C2(CC2)C(F)(F)F)cc1 Show InChI InChI=1S/C21H23F6N3O3/c22-20(23,24)19(5-6-19)16(31)29-10-7-18(8-11-29)9-12-30(13-18)17(32)28-14-1-3-15(4-2-14)33-21(25,26)27/h1-4H,5-13H2,(H,28,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... |
Bioorg Med Chem Lett 23: 5975-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.054
BindingDB Entry DOI: 10.7270/Q2TT4SDR |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50441474

(CHEMBL2436563)Show SMILES Fc1cccc(F)c1C(=O)N1CCC2(CCN(C2)C(=O)Nc2ccc(cc2)C(F)(F)F)CC1 Show InChI InChI=1S/C23H22F5N3O2/c24-17-2-1-3-18(25)19(17)20(32)30-11-8-22(9-12-30)10-13-31(14-22)21(33)29-16-6-4-15(5-7-16)23(26,27)28/h1-7H,8-14H2,(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... |
Bioorg Med Chem Lett 23: 5975-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.054
BindingDB Entry DOI: 10.7270/Q2TT4SDR |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50327848
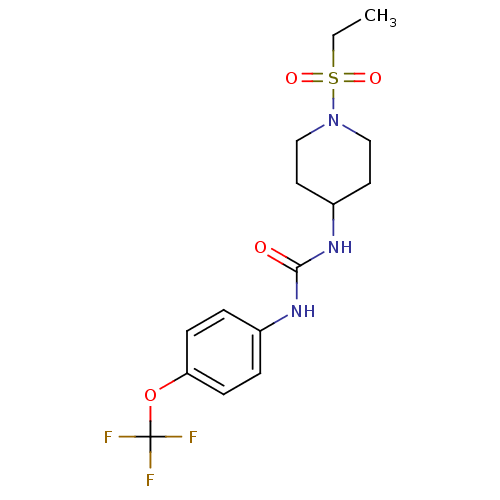
(1-(1-(Ethylsulfonyl)piperidin-4-yl)-3-(4-(trifluor...)Show SMILES CCS(=O)(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C15H20F3N3O4S/c1-2-26(23,24)21-9-7-12(8-10-21)20-14(22)19-11-3-5-13(6-4-11)25-15(16,17)18/h3-6,12H,2,7-10H2,1H3,(H2,19,20,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Entomology and University of California Davis Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase by fluorescence assay |
J Med Chem 53: 7067-75 (2010)
Article DOI: 10.1021/jm100691c
BindingDB Entry DOI: 10.7270/Q2GH9J6V |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50327850
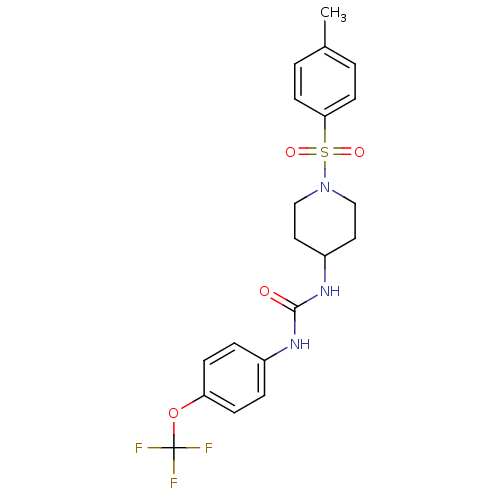
(1-(1-Tosylpiperidin-4-yl)-3-(4-(trifluoromethoxy)p...)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C20H22F3N3O4S/c1-14-2-8-18(9-3-14)31(28,29)26-12-10-16(11-13-26)25-19(27)24-15-4-6-17(7-5-15)30-20(21,22)23/h2-9,16H,10-13H2,1H3,(H2,24,25,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Entomology and University of California Davis Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase by fluorescence assay |
J Med Chem 53: 7067-75 (2010)
Article DOI: 10.1021/jm100691c
BindingDB Entry DOI: 10.7270/Q2GH9J6V |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591336

(CHEMBL5179027)Show SMILES ClC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)C1CC1 |TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591337
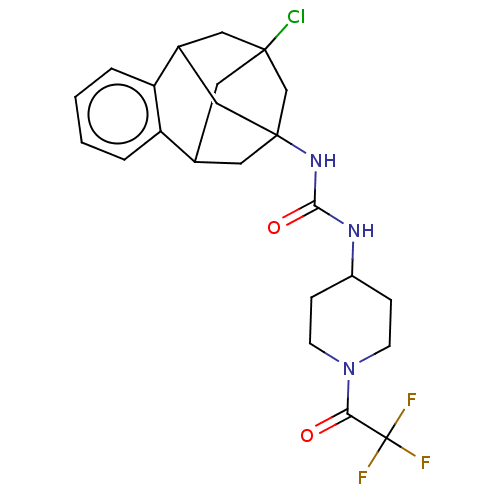
(CHEMBL5192445)Show SMILES FC(F)(F)C(=O)N1CCC(CC1)NC(=O)NC12CC3CC(Cl)(CC(C1)c1ccccc31)C2 |TLB:30:18:23.24.22:31,25:23:18.19.17:31,26:25:24:20.22.31,21:20:24:30.25.18.17,THB:19:18:24:20.22.31,19:20:24:30.25.18.17| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591340
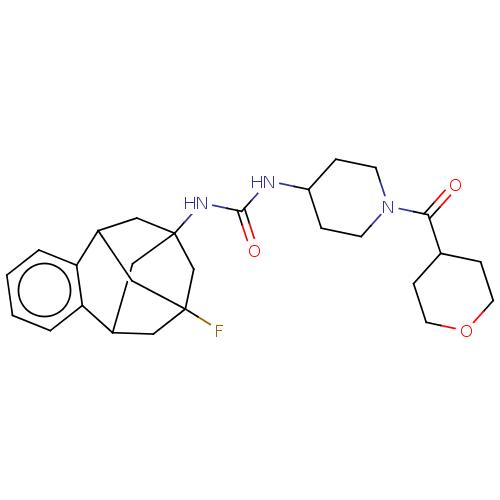
(CHEMBL5203787)Show SMILES FC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)C1CCOCC1 |TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591341
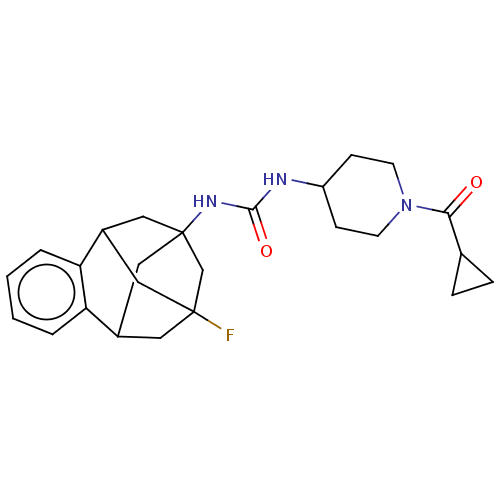
(CHEMBL5177372)Show SMILES FC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)C1CC1 |TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591344
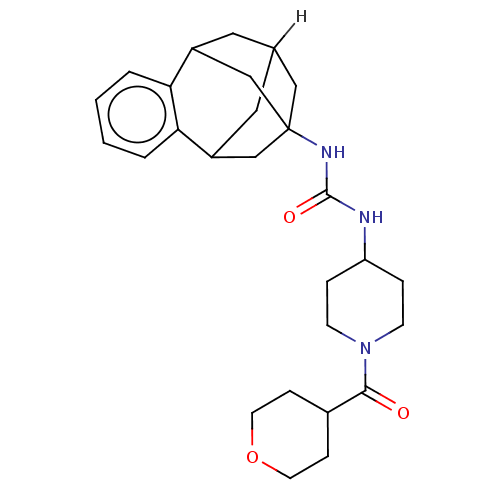
(CHEMBL5208857)Show SMILES [2H]C12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)C1CCOCC1 |TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50604188
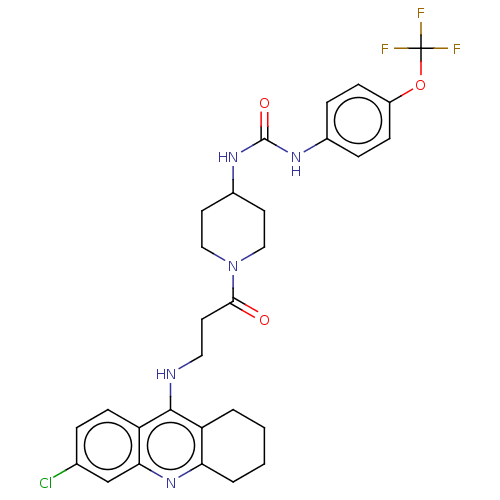
(CHEMBL5204900)Show SMILES FC(F)(F)Oc1ccc(NC(=O)NC2CCN(CC2)C(=O)CCNc2c3CCCCc3nc3cc(Cl)ccc23)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02150
BindingDB Entry DOI: 10.7270/Q2F76HMM |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50604191
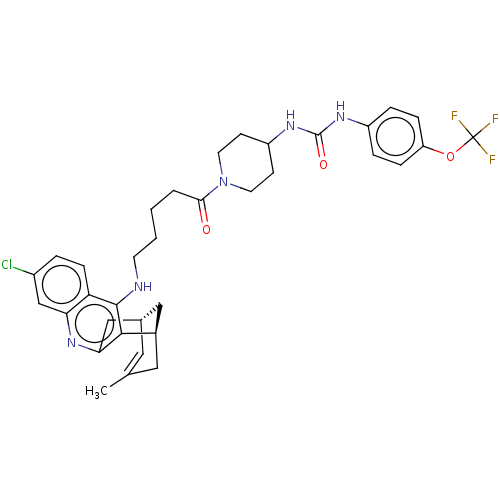
(CHEMBL5207628)Show SMILES [H][C@]12Cc3nc4cc(Cl)ccc4c(NCCCCC(=O)N4CCC(CC4)NC(=O)Nc4ccc(OC(F)(F)F)cc4)c3[C@]([H])(CC(C)=C1)C2 |r,c:50| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02150
BindingDB Entry DOI: 10.7270/Q2F76HMM |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50264113
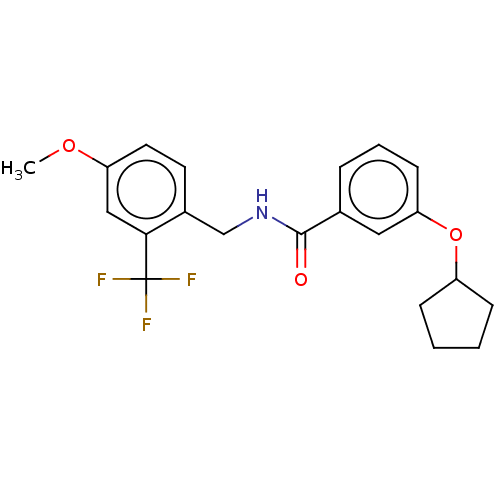
(CHEMBL4099452)Show SMILES COc1ccc(CNC(=O)c2cccc(OC3CCCC3)c2)c(c1)C(F)(F)F Show InChI InChI=1S/C21H22F3NO3/c1-27-17-10-9-15(19(12-17)21(22,23)24)13-25-20(26)14-5-4-8-18(11-14)28-16-6-2-3-7-16/h4-5,8-12,16H,2-3,6-7,13H2,1H3,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sEH using MNPC as substrate by fluorescence-based assay |
J Med Chem 61: 3541-3550 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01804
BindingDB Entry DOI: 10.7270/Q2ZP48KN |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50264152
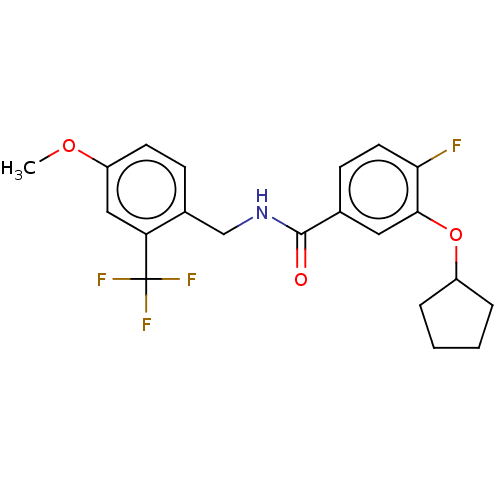
(CHEMBL4072536)Show SMILES COc1ccc(CNC(=O)c2ccc(F)c(OC3CCCC3)c2)c(c1)C(F)(F)F Show InChI InChI=1S/C21H21F4NO3/c1-28-16-8-6-14(17(11-16)21(23,24)25)12-26-20(27)13-7-9-18(22)19(10-13)29-15-4-2-3-5-15/h6-11,15H,2-5,12H2,1H3,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sEH using MNPC as substrate by fluorescence-based assay |
J Med Chem 61: 3541-3550 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01804
BindingDB Entry DOI: 10.7270/Q2ZP48KN |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM158481

(US9029401, 1728 (t-TUCB))Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)Nc2ccc(OC(F)(F)F)cc2)cc1 |r,wU:8.7,wD:11.14,(10,,;8.67,-.77,;8.67,-2.31,;7.34,,;6,-.77,;4.67,,;4.67,1.54,;3.33,2.31,;2,1.54,;2,,;.67,-.77,;-.67,,;-.67,1.54,;.67,2.31,;-2,-.77,;-3.33,,;-3.33,1.54,;-4.67,-.77,;-6,,;-6,1.54,;-7.34,2.31,;-8.67,1.54,;-10,2.31,;-10,3.85,;-10,5.39,;-8.67,4.62,;-11.34,4.62,;-8.67,,;-7.34,-.77,;6,2.31,;7.34,1.54,)| Show InChI InChI=1S/C21H21F3N2O5/c22-21(23,24)31-18-11-5-15(6-12-18)26-20(29)25-14-3-9-17(10-4-14)30-16-7-1-13(2-8-16)19(27)28/h1-2,5-8,11-12,14,17H,3-4,9-10H2,(H,27,28)(H2,25,26,29)/t14-,17- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase (unknown origin) |
Bioorg Med Chem Lett 28: 762-768 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.003
BindingDB Entry DOI: 10.7270/Q2PV6NZ4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50327846
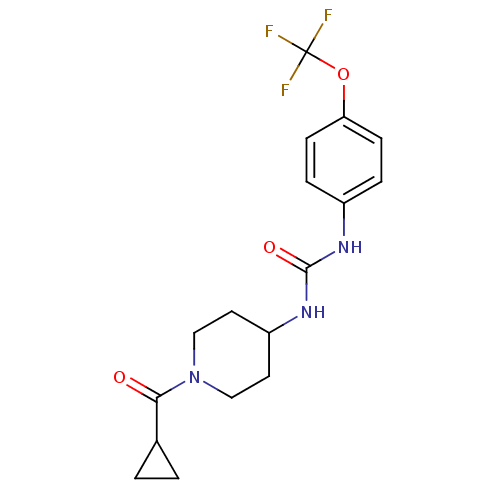
(1-(1-(Cyclopropanecarbonyl)piperidin-4-yl)-3-(4-(t...)Show SMILES FC(F)(F)Oc1ccc(NC(=O)NC2CCN(CC2)C(=O)C2CC2)cc1 Show InChI InChI=1S/C17H20F3N3O3/c18-17(19,20)26-14-5-3-12(4-6-14)21-16(25)22-13-7-9-23(10-8-13)15(24)11-1-2-11/h3-6,11,13H,1-2,7-10H2,(H2,21,22,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase (unknown origin) |
Medchemcomm 379-384 (2012)
Article DOI: 10.1039/c2md00288d
BindingDB Entry DOI: 10.7270/Q2JD50RG |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50496759

(CHEMBL3222130)Show SMILES CC(C)(C)OC(=O)N1CCN(CCCCOc2cccc(NC(=O)CC34CC5CC(CC(C5)C3)C4)c2)CC1 |TLB:24:25:28:32.31.30,THB:26:27:30:34.25.33,26:25:28.27.32:30,33:25:28:32.31.30,33:31:28:34.26.25| Show InChI InChI=1S/C31H47N3O4/c1-30(2,3)38-29(36)34-12-10-33(11-13-34)9-4-5-14-37-27-8-6-7-26(18-27)32-28(35)22-31-19-23-15-24(20-31)17-25(16-23)21-31/h6-8,18,23-25H,4-5,9-17,19-22H2,1-3H3,(H,32,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a... |
Medchemcomm 379-384 (2012)
Article DOI: 10.1039/c2md00288d
BindingDB Entry DOI: 10.7270/Q2JD50RG |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50351247
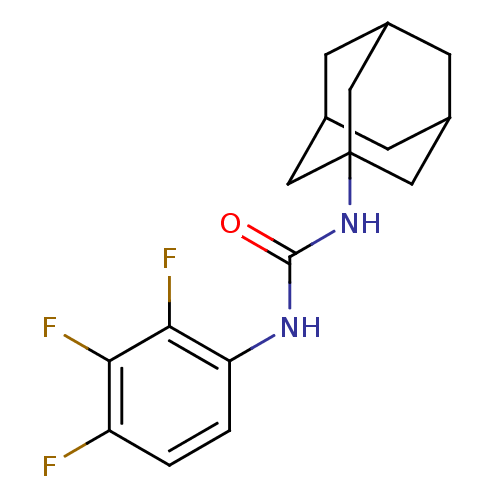
(CHEMBL1818385)Show SMILES Fc1ccc(NC(=O)NC23CC4CC(CC(C4)C2)C3)c(F)c1F |TLB:8:9:12:16.14.15,THB:14:13:10:16.15.17,14:15:12.13.18:10,17:15:12:18.9.10,17:9:12:16.14.15| Show InChI InChI=1S/C17H19F3N2O/c18-12-1-2-13(15(20)14(12)19)21-16(23)22-17-6-9-3-10(7-17)5-11(4-9)8-17/h1-2,9-11H,3-8H2,(H2,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human liver soluble epoxide hydrolase expressed in baculovirus infected Sf21 cells using NEPC as substrate by fluorescence ... |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.115078
BindingDB Entry DOI: 10.7270/Q2668HMV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50351248

(CHEMBL1818384)Show SMILES Fc1ccc(NC(=O)NC2C3CC4CC(C3)CC2C4)c(F)c1F |TLB:18:17:15:11.12.13,THB:8:9:15:11.12.13,18:12:9.17.16:15,13:12:9:16.14.15,13:14:9:11.18.12,(-9.43,3.25,;-8.09,2.48,;-6.76,3.25,;-5.43,2.49,;-5.42,.94,;-4.09,.17,;-2.75,.94,;-2.76,2.48,;-1.42,.17,;-.09,.95,;1.13,2.21,;2.44,1.7,;3.84,2.02,;3.88,3.55,;2.49,4.15,;1.14,3.69,;1.43,2.93,;1.41,1.35,;2.82,.76,;-6.76,.17,;-6.76,-1.37,;-8.09,.94,;-9.43,.17,)| Show InChI InChI=1S/C17H19F3N2O/c18-12-1-2-13(15(20)14(12)19)21-17(23)22-16-10-4-8-3-9(6-10)7-11(16)5-8/h1-2,8-11,16H,3-7H2,(H2,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human liver soluble epoxide hydrolase expressed in baculovirus infected Sf21 cells using NEPC as substrate by fluorescence ... |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.115078
BindingDB Entry DOI: 10.7270/Q2668HMV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50525808
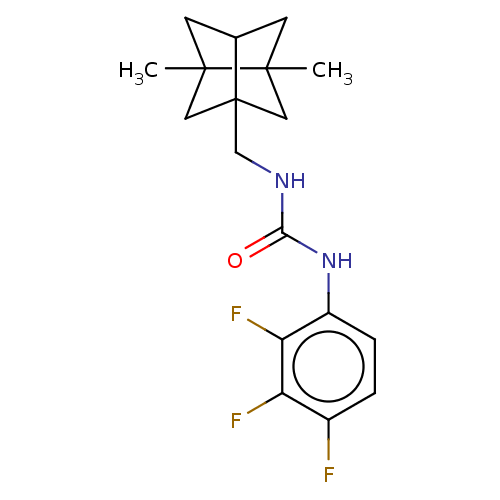
(CHEMBL4454212)Show SMILES CC12CC3CC1(C)CC3(CNC(=O)Nc1ccc(F)c(F)c1F)C2 |TLB:7:5:23.8:2,THB:7:8:4.5:2,6:5:23.8:2| Show InChI InChI=1S/C18H21F3N2O/c1-16-5-10-6-17(16,2)8-18(10,7-16)9-22-15(24)23-12-4-3-11(19)13(20)14(12)21/h3-4,10H,5-9H2,1-2H3,(H2,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human liver soluble epoxide hydrolase expressed in baculovirus infected Sf21 cells using NEPC as substrate by fluorescence ... |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.115078
BindingDB Entry DOI: 10.7270/Q2668HMV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50539356
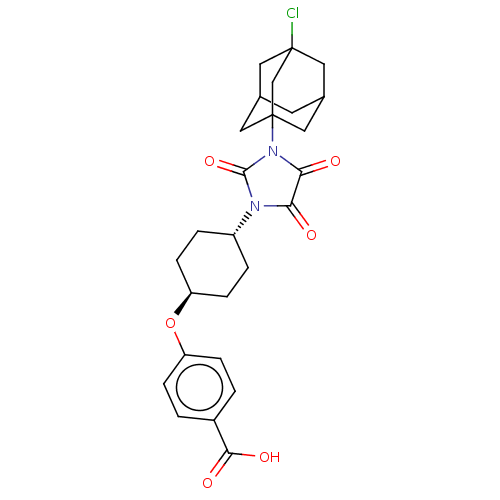
(CHEMBL4641404)Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)N2C(=O)N(C(=O)C2=O)C23CC4CC(CC(Cl)(C4)C2)C3)cc1 |r,wU:8.7,wD:11.14,TLB:32:22:30:27.25.26,25:26:30.24.23:31,THB:32:26:30:23.22.31,17:22:30:27.25.26,25:24:31:27.26.32,(46.15,-13.46,;44.82,-12.69,;44.81,-11.15,;43.49,-13.47,;42.15,-12.71,;40.82,-13.48,;40.82,-15.02,;39.49,-15.79,;38.15,-15.02,;36.83,-15.78,;35.49,-15,;35.5,-13.46,;36.84,-12.7,;38.16,-13.47,;34.17,-12.69,;32.76,-13.3,;32.44,-14.81,;31.74,-12.16,;32.51,-10.83,;31.89,-9.42,;34.02,-11.15,;35.17,-10.13,;30.2,-12.19,;29.04,-11.06,;27.46,-10.95,;26.79,-12.31,;27.99,-13.42,;27.75,-12,;28.34,-10.71,;28.73,-9.22,;27.15,-9.42,;29.82,-10.81,;29.52,-13.54,;42.16,-15.79,;43.49,-15.02,)| Show InChI InChI=1S/C26H29ClN2O6/c27-25-10-15-9-16(11-25)13-26(12-15,14-25)29-22(31)21(30)28(24(29)34)18-3-7-20(8-4-18)35-19-5-1-17(2-6-19)23(32)33/h1-2,5-6,15-16,18,20H,3-4,7-14H2,(H,32,33)/t15?,16?,18-,20-,25?,26? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126908
BindingDB Entry DOI: 10.7270/Q2M0490F |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50539380
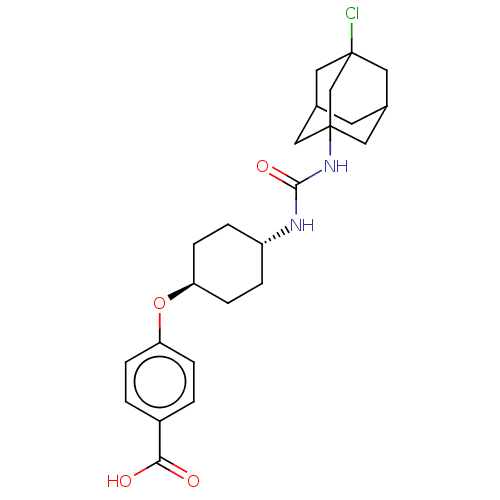
(CHEMBL4643551)Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(Cl)(C4)C2)C3)cc1 |r,wU:8.7,wD:11.14,TLB:21:22:26.20.19:27,28:18:26:23.21.22,THB:17:18:26:23.21.22,21:20:27:23.22.28,28:22:26:19.18.27,(68.54,-12.24,;67.2,-11.47,;67.19,-9.93,;65.85,-12.25,;64.52,-11.49,;63.2,-12.26,;63.19,-13.8,;61.86,-14.59,;60.5,-13.83,;59.18,-14.61,;57.83,-13.86,;57.8,-12.32,;59.16,-11.51,;60.49,-12.28,;56.43,-11.59,;55.12,-12.42,;55.19,-13.97,;53.75,-11.71,;52.39,-12.42,;50.86,-11.93,;49.4,-12.53,;49.41,-14.04,;50.97,-14.5,;50.12,-13.34,;50.07,-11.93,;49.76,-10.42,;48.44,-11.3,;51.44,-11.36,;52.38,-13.93,;64.53,-14.57,;65.86,-13.8,)| Show InChI InChI=1S/C24H31ClN2O4/c25-23-10-15-9-16(11-23)13-24(12-15,14-23)27-22(30)26-18-3-7-20(8-4-18)31-19-5-1-17(2-6-19)21(28)29/h1-2,5-6,15-16,18,20H,3-4,7-14H2,(H,28,29)(H2,26,27,30)/t15?,16?,18-,20-,23?,24? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using cyano(2methoxy naphthalen-6-yl)methyl trans-(3-phenyloxyran-2-yl)methylcarbonate as substrate pre... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126908
BindingDB Entry DOI: 10.7270/Q2M0490F |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50546449

(CHEMBL4746902) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127430
BindingDB Entry DOI: 10.7270/Q2WQ07D6 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50546457

(CHEMBL4786305)Show SMILES O=C(NCCCCCCCCCCNC(=O)NC1CC2CC1C=C2)NC1CC2CC1C=C2 |c:23,33| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127430
BindingDB Entry DOI: 10.7270/Q2WQ07D6 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50546459

(CHEMBL4759033)Show SMILES O=C(NCCCCCCCCNC(=O)NC1CC2CCC1C2)NC1CC2CCC1C2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127430
BindingDB Entry DOI: 10.7270/Q2WQ07D6 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50546461
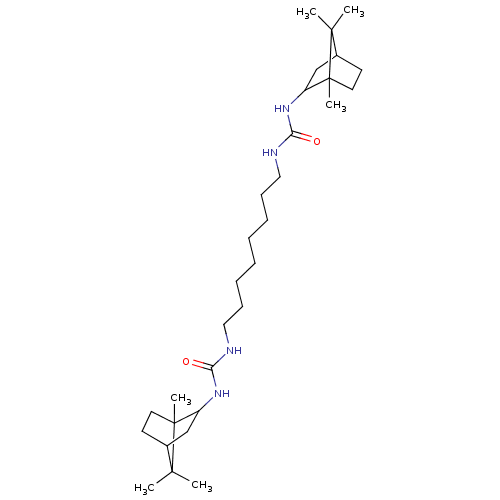
(CHEMBL4750771)Show SMILES CC1(C)C2CCC1(C)C(C2)NC(=O)NCCCCCCCCNC(=O)NC1CC2CCC1(C)C2(C)C | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127430
BindingDB Entry DOI: 10.7270/Q2WQ07D6 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50577862

(CHEMBL4869434)Show SMILES CC12CC3CC(C)(C1)CC(C3)(C2)NC(=O)Nc1ccc(cc1)C(=O)N1CCCCC1 |TLB:10:9:7:4.3.2,12:9:7:4.3.2,12:9:7.5.4:2,THB:8:5:2:11.9.10,8:9:7.5.4:2,10:3:7:11.8.9,6:5:2:11.9.10| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant sEH using PHOME as substrate measured after 10 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113678
BindingDB Entry DOI: 10.7270/Q2FF3X64 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50577868
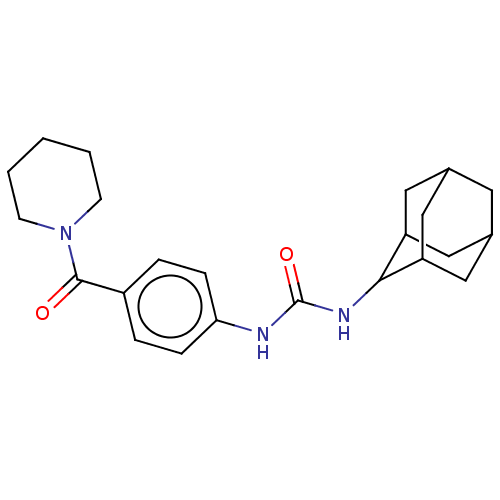
(CHEMBL4872738)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)Nc1ccc(cc1)C(=O)N1CCCCC1 |TLB:2:3:5:9.8.7,THB:7:6:3:9.8.10,7:8:3:5.6.12,10:11:5:9.8.7,10:8:5:3.12.11,(7.72,-25.81,;7.79,-27.34,;6.48,-28.18,;5.12,-27.47,;3.76,-28.13,;3.06,-29.52,;2.92,-27.77,;1.81,-26.77,;2.37,-25.46,;2.58,-27.06,;3.74,-24.81,;4.86,-25.85,;4.17,-27.12,;9.15,-28.06,;10.45,-27.23,;11.81,-27.94,;13.11,-27.11,;13.04,-25.57,;11.67,-24.86,;10.38,-25.7,;14.34,-24.74,;14.27,-23.21,;15.71,-25.45,;15.77,-27,;17.13,-27.71,;18.43,-26.88,;18.36,-25.34,;17,-24.63,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant sEH using PHOME as substrate measured after 10 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113678
BindingDB Entry DOI: 10.7270/Q2FF3X64 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50581727
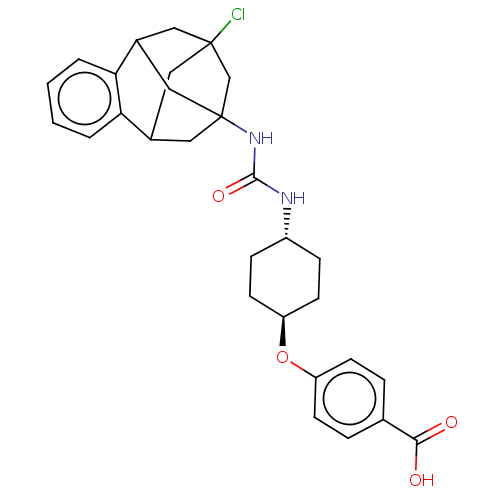
(CHEMBL5084744)Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(Cl)(CC(C2)c2ccccc42)C3)cc1 |r,wU:8.7,wD:11.14,TLB:32:20:25.26.24:33,28:27:19:21.22.33,31:32:19:21.22.33,THB:24:25:19:21.22.33,24:22:19:32.25.26.27,27:25:20.19.21:33,23:22:19:32.25.26.27,(49.77,-46.61,;49.78,-45.07,;51.11,-44.3,;48.45,-44.3,;47.11,-45.06,;45.78,-44.28,;45.79,-42.75,;44.46,-41.97,;43.13,-42.73,;43.12,-44.28,;41.79,-45.04,;40.46,-44.26,;40.45,-42.72,;41.79,-41.96,;39.12,-45.03,;37.8,-44.27,;37.8,-42.74,;36.49,-45.03,;35.17,-44.27,;34.17,-45.53,;32.78,-44.97,;32.77,-43.4,;33.8,-42.18,;33.4,-40.69,;32.46,-42.65,;32.47,-44.12,;33.79,-44.61,;30.19,-44.31,;28.71,-44.72,;28.32,-46.22,;29.44,-47.3,;30.92,-46.88,;31.28,-45.39,;35.18,-42.76,;47.12,-41.98,;48.45,-42.75,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409005
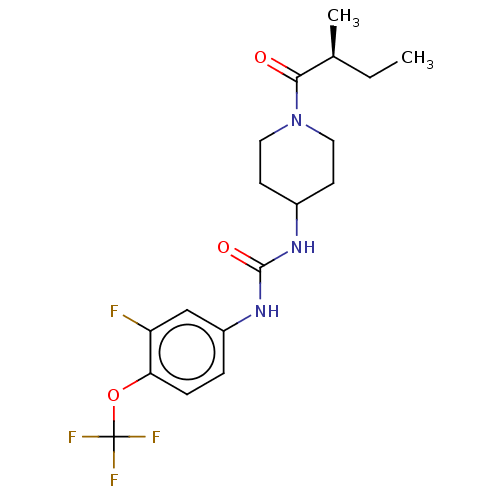
(US10377744, Compound No. 26 | US11123311, Compound...)Show SMILES CC[C@H](C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)c(F)c1 |r| Show InChI InChI=1S/C18H23F4N3O3/c1-3-11(2)16(26)25-8-6-12(7-9-25)23-17(27)24-13-4-5-15(14(19)10-13)28-18(20,21)22/h4-5,10-12H,3,6-9H2,1-2H3,(H2,23,24,27)/t11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50593497

(CHEMBL5180183)Show SMILES O=C(NC1=CC=CC1)NC12CC3CC(CC(C3)C1)C2 |c:5,t:3,TLB:8:9:12.11.16:14,THB:10:11:14:18.9.17,10:9:12.11.16:14,17:9:12:16.15.14,17:15:12:18.10.9,8:9:12:16.15.14| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409005

(US10377744, Compound No. 26 | US11123311, Compound...)Show SMILES CC[C@H](C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)c(F)c1 |r| Show InChI InChI=1S/C18H23F4N3O3/c1-3-11(2)16(26)25-8-6-12(7-9-25)23-17(27)24-13-4-5-15(14(19)10-13)28-18(20,21)22/h4-5,10-12H,3,6-9H2,1-2H3,(H2,23,24,27)/t11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data