 Found 194 hits of kd for UniProtKB: P29317
Found 194 hits of kd for UniProtKB: P29317 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PCBioAssay
| n/a | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2513WK1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHA2 kinase domain |
Nat Biotechnol 26: 127-32 (2008)
Article DOI: 10.1038/nbt1358
BindingDB Entry DOI: 10.7270/Q2TT4RX2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHA2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Kinobeads competition assays were performed in 96-well format as previously described using mixed protein lysates of four cancer cell lines (K-562, M... |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50355504
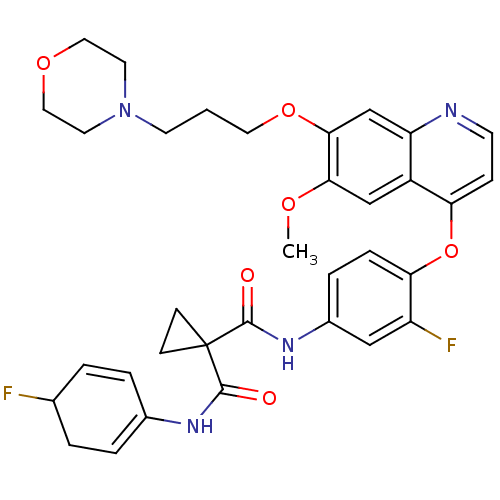
(CHEMBL1908393)Show SMILES COc1cc2c(Oc3ccc(NC(=O)C4(CC4)C(=O)NC4=CCC(F)C=C4)cc3F)ccnc2cc1OCCCN1CCOCC1 |c:26,t:21| Show InChI InChI=1S/C34H36F2N4O6/c1-43-30-20-25-27(21-31(30)45-16-2-13-40-14-17-44-18-15-40)37-12-9-28(25)46-29-8-7-24(19-26(29)36)39-33(42)34(10-11-34)32(41)38-23-5-3-22(35)4-6-23/h3,5-9,12,19-22H,2,4,10-11,13-18H2,1H3,(H,38,41)(H,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHA2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM6568

(6-(2,6-dichlorophenyl)-8-methyl-2-{[3-(methylsulfa...)Show SMILES CSc1cccc(Nc2ncc3cc(-c4c(Cl)cccc4Cl)c(=O)n(C)c3n2)c1 |(-12.18,1.86,;-10.84,1.09,;-9.51,1.86,;-9.51,3.4,;-8.18,4.17,;-6.84,3.4,;-6.84,1.86,;-5.51,1.08,;-4.18,1.85,;-4.18,3.39,;-2.84,4.16,;-1.51,3.4,;-.18,4.17,;1.16,3.4,;2.49,4.17,;2.49,5.71,;1.16,6.48,;3.83,6.48,;5.16,5.71,;5.16,4.17,;3.83,3.39,;3.83,1.85,;1.16,1.86,;2.49,1.09,;-.18,1.09,;-.18,-.45,;-1.51,1.86,;-2.84,1.08,;-8.18,1.08,)| Show InChI InChI=1S/C21H16Cl2N4OS/c1-27-19-12(9-15(20(27)28)18-16(22)7-4-8-17(18)23)11-24-21(26-19)25-13-5-3-6-14(10-13)29-2/h3-11H,1-2H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHA2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Kinobeads competition assays were performed in 96-well format as previously described using mixed protein lysates of four cancer cell lines (K-562, M... |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM26145
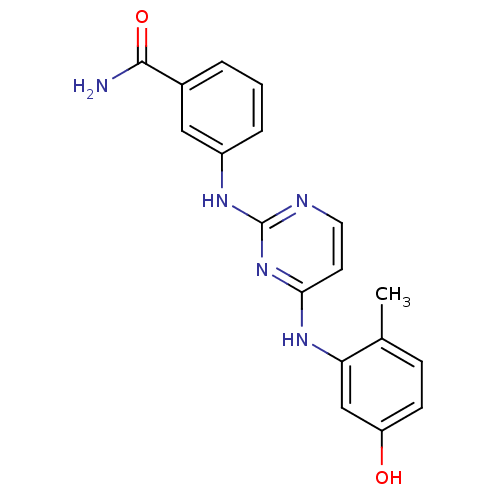
(2,4-dianilino pyrimidine, 2 | 3-({4-[(5-hydroxy-2-...)Show InChI InChI=1S/C18H17N5O2/c1-11-5-6-14(24)10-15(11)22-16-7-8-20-18(23-16)21-13-4-2-3-12(9-13)17(19)25/h2-10,24H,1H3,(H2,19,25)(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human EPHA2 |
J Med Chem 51: 7898-914 (2008)
Article DOI: 10.1021/jm8011036
BindingDB Entry DOI: 10.7270/Q2WS8T4C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM209858
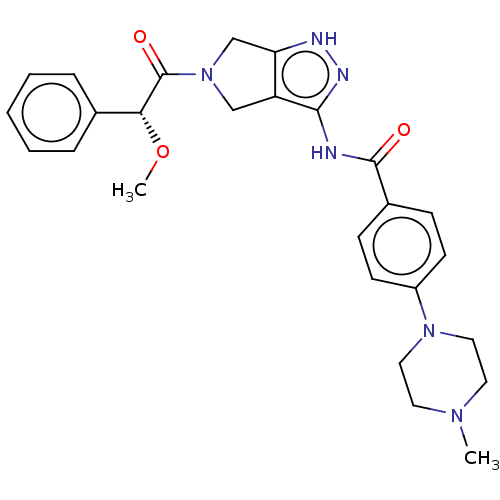
(Danusertib | N-[5-[(2R)-2-methoxy-2-phenylacetyl]-...)Show SMILES CO[C@@H](C(=O)N1Cc2[nH]nc(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1)c1ccccc1 Show InChI InChI=1S/C26H30N6O3/c1-30-12-14-31(15-13-30)20-10-8-19(9-11-20)25(33)27-24-21-16-32(17-22(21)28-29-24)26(34)23(35-2)18-6-4-3-5-7-18/h3-11,23H,12-17H2,1-2H3,(H2,27,28,29,33)/t23-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Kinobeads competition assays were performed in 96-well format as previously described using mixed protein lysates of four cancer cell lines (K-562, M... |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50382959

(CEP-32496 | CHEMBL2029988 | US9730937, Example 261)Show SMILES COc1cc2ncnc(Oc3cccc(NC(=O)Nc4cc(on4)C(C)(C)C(F)(F)F)c3)c2cc1OC Show InChI InChI=1S/C24H22F3N5O5/c1-23(2,24(25,26)27)19-11-20(32-37-19)31-22(33)30-13-6-5-7-14(8-13)36-21-15-9-17(34-3)18(35-4)10-16(15)28-12-29-21/h5-12H,1-4H3,(H2,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of EPHA2 in human HEK293 cells after 1 hr by competition binding assay |
J Med Chem 55: 1082-105 (2012)
Article DOI: 10.1021/jm2009925
BindingDB Entry DOI: 10.7270/Q2571D2T |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM4552

(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHA2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588606
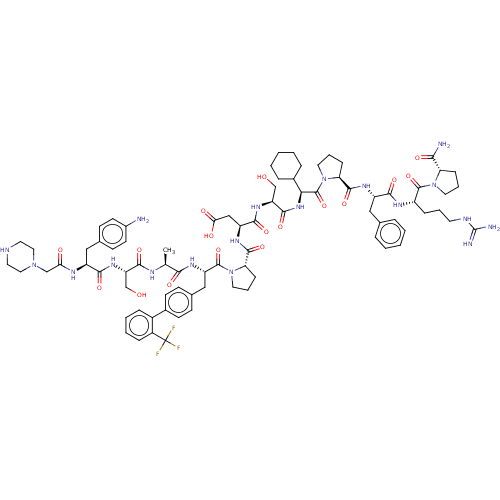
(CHEMBL5179135)Show SMILES C[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(N)cc1)NC(=O)CN1CCNCC1)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1C(F)(F)F)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM209859
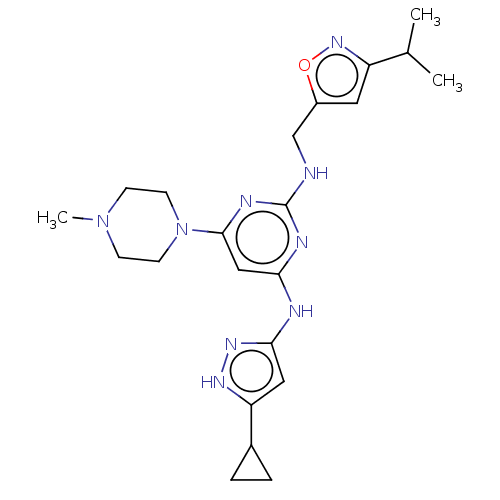
(4-N-(5-cyclopropyl-1H-pyrazol-3-yl)-6-(4-methylpip...)Show SMILES CC(C)c1cc(CNc2nc(Nc3cc([nH]n3)C3CC3)cc(n2)N2CCN(C)CC2)on1 Show InChI InChI=1S/C22H31N9O/c1-14(2)17-10-16(32-29-17)13-23-22-25-19(24-20-11-18(27-28-20)15-4-5-15)12-21(26-22)31-8-6-30(3)7-9-31/h10-12,14-15H,4-9,13H2,1-3H3,(H3,23,24,25,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Kinobeads competition assays were performed in 96-well format as previously described using mixed protein lysates of four cancer cell lines (K-562, M... |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Kinobeads competition assays were performed in 96-well format as previously described using mixed protein lysates of four cancer cell lines (K-562, M... |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588591
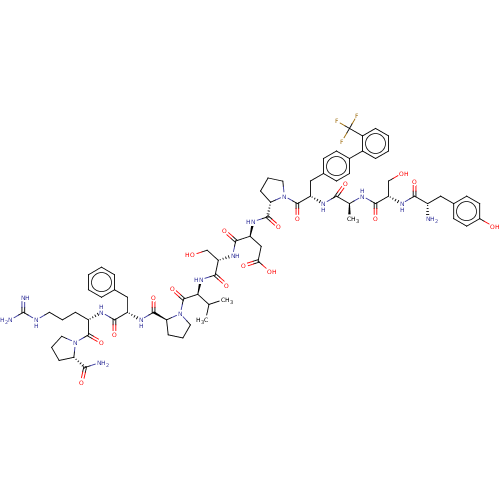
(CHEMBL5175743)Show SMILES CC(C)[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1C(F)(F)F)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588608
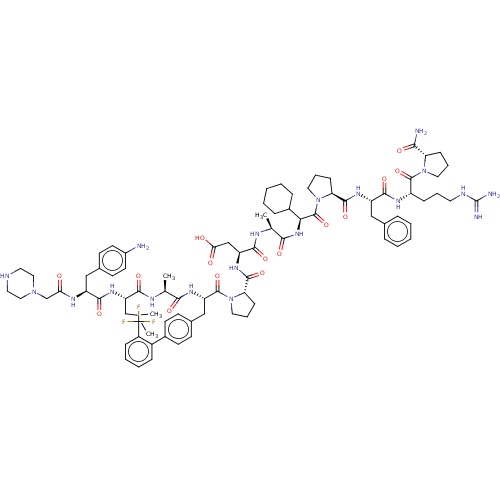
(CHEMBL5192123)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc(N)cc1)NC(=O)CN1CCNCC1)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1C(F)(F)F)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588607
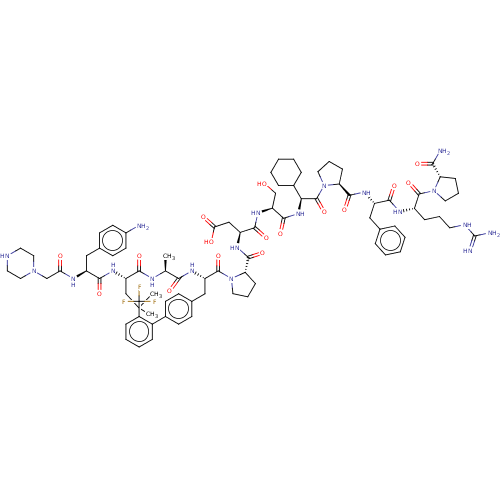
(CHEMBL5177810)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc(N)cc1)NC(=O)CN1CCNCC1)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1C(F)(F)F)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM50100615
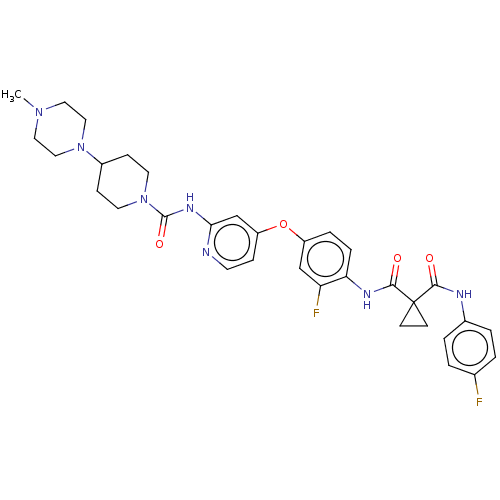
(E-7050 | E7050 | Golvatinib)Show SMILES CN1CCN(CC1)C1CCN(CC1)C(=O)Nc1cc(Oc2ccc(NC(=O)C3(CC3)C(=O)Nc3ccc(F)cc3)c(F)c2)ccn1 Show InChI InChI=1S/C33H37F2N7O4/c1-40-16-18-41(19-17-40)24-9-14-42(15-10-24)32(45)39-29-21-26(8-13-36-29)46-25-6-7-28(27(35)20-25)38-31(44)33(11-12-33)30(43)37-23-4-2-22(34)3-5-23/h2-8,13,20-21,24H,9-12,14-19H2,1H3,(H,37,43)(H,38,44)(H,36,39,45) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Kinobeads competition assays were performed in 96-well format as previously described using mixed protein lysates of four cancer cell lines (K-562, M... |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM4552

(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Kinobeads competition assays were performed in 96-well format as previously described using mixed protein lysates of four cancer cell lines (K-562, M... |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588592
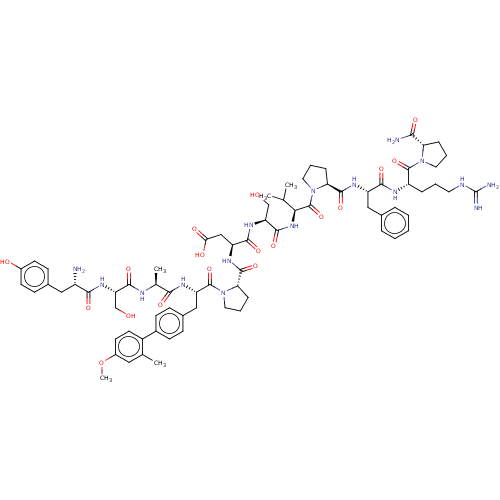
(CHEMBL5180572)Show SMILES COc1ccc(c(C)c1)-c1ccc(C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2CCC[C@H]2C(N)=O)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM6568

(6-(2,6-dichlorophenyl)-8-methyl-2-{[3-(methylsulfa...)Show SMILES CSc1cccc(Nc2ncc3cc(-c4c(Cl)cccc4Cl)c(=O)n(C)c3n2)c1 |(-12.18,1.86,;-10.84,1.09,;-9.51,1.86,;-9.51,3.4,;-8.18,4.17,;-6.84,3.4,;-6.84,1.86,;-5.51,1.08,;-4.18,1.85,;-4.18,3.39,;-2.84,4.16,;-1.51,3.4,;-.18,4.17,;1.16,3.4,;2.49,4.17,;2.49,5.71,;1.16,6.48,;3.83,6.48,;5.16,5.71,;5.16,4.17,;3.83,3.39,;3.83,1.85,;1.16,1.86,;2.49,1.09,;-.18,1.09,;-.18,-.45,;-1.51,1.86,;-2.84,1.08,;-8.18,1.08,)| Show InChI InChI=1S/C21H16Cl2N4OS/c1-27-19-12(9-15(20(27)28)18-16(22)7-4-8-17(18)23)11-24-21(26-19)25-13-5-3-6-14(10-13)29-2/h3-11H,1-2H3,(H,24,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Kinobeads competition assays were performed in 96-well format as previously described using mixed protein lysates of four cancer cell lines (K-562, M... |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM50399540

(FORETINIB | US10464902, Foretinib | US10882853, Co...)Show SMILES COc1cc2c(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)ccnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C34H34F2N4O6/c1-43-30-20-25-27(21-31(30)45-16-2-13-40-14-17-44-18-15-40)37-12-9-28(25)46-29-8-7-24(19-26(29)36)39-33(42)34(10-11-34)32(41)38-23-5-3-22(35)4-6-23/h3-9,12,19-21H,2,10-11,13-18H2,1H3,(H,38,41)(H,39,42) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Kinobeads competition assays were performed in 96-well format as previously described using mixed protein lysates of four cancer cell lines (K-562, M... |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588590
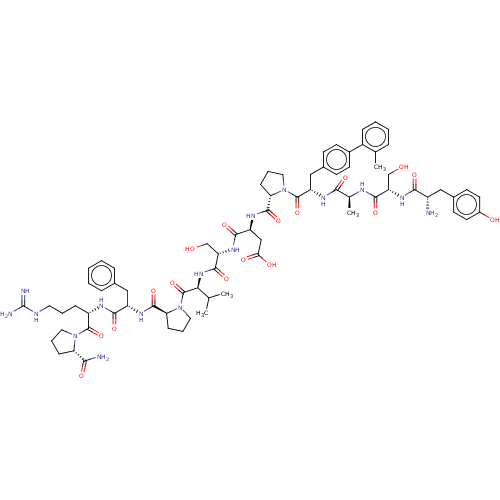
(CHEMBL5202336)Show SMILES CC(C)[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588603

(CHEMBL5202033)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50246750

(CHEMBL4071045)Show SMILES NC(=O)[C@H](CCCCNC(=S)Nc1ccc(c(c1)C(O)=O)-c1c2ccc(O)cc2oc2cc(=O)ccc12)NC(=O)CN1C[C@H](Cc2ccccc2)NC(=O)CN(C[C@H](CCC(O)=O)NC(=O)CN(C[C@H](CCC(O)=O)NC(=O)CN(C[C@H](Cc2ccccc2)NC(=O)c2ccc(CSCCC1=O)cc2)C(=O)CCC1CCCCC1)C(=O)Cc1ccc2OCOc2c1)C(=O)CCC(O)=O |r,wU:69.74,57.62,81.86,43.47,3.40,(24.07,-26.19,;22.75,-25.4,;21.41,-26.16,;22.76,-23.86,;24.1,-23.11,;25.43,-23.89,;26.77,-23.13,;28.1,-23.91,;29.44,-23.15,;30.76,-23.94,;30.75,-25.48,;32.1,-23.18,;33.43,-23.96,;34.76,-23.2,;36.09,-23.97,;36.08,-25.52,;34.74,-26.28,;33.41,-25.5,;33.95,-27.6,;34.71,-28.95,;32.41,-27.59,;37.4,-26.3,;38.74,-25.54,;38.75,-24.01,;40.09,-23.25,;41.42,-24.03,;42.76,-23.27,;41.4,-25.57,;40.06,-26.32,;40.05,-27.87,;38.71,-28.62,;38.69,-30.15,;37.35,-30.89,;37.32,-32.43,;36.04,-30.1,;36.05,-28.6,;37.39,-27.83,;21.43,-23.08,;21.45,-21.54,;22.79,-20.78,;20.12,-20.76,;20.14,-19.22,;21.47,-20,;22.73,-19.23,;24.17,-19.94,;25.64,-19.48,;26.76,-20.53,;28.23,-20.07,;28.57,-18.57,;27.43,-17.52,;25.96,-17.99,;22.62,-17.2,;23.95,-16.42,;25.29,-17.18,;23.94,-14.88,;22.6,-14.12,;22.58,-12.58,;21.24,-11.82,;21.23,-10.28,;22.56,-9.5,;22.55,-7.96,;21.21,-7.2,;23.87,-7.18,;19.92,-12.6,;18.58,-11.85,;18.56,-10.31,;17.25,-12.63,;15.91,-11.87,;14.58,-12.65,;13.24,-11.89,;13.23,-10.35,;11.89,-9.59,;11.88,-8.05,;13.2,-7.27,;10.54,-7.29,;11.92,-12.67,;10.58,-11.91,;10.56,-10.37,;9.25,-12.69,;9.26,-14.23,;8.16,-15.32,;8.15,-16.86,;6.81,-17.63,;5.48,-16.85,;5.5,-15.31,;4.17,-14.53,;2.83,-15.3,;2.83,-16.84,;4.16,-17.61,;9.48,-17.64,;9.47,-19.18,;8.14,-19.94,;10.8,-19.96,;10.79,-21.49,;12.12,-22.27,;13.46,-21.51,;14.79,-22.28,;16.13,-21.52,;16.13,-19.98,;17.47,-19.21,;18.8,-19.99,;18.79,-21.53,;13.46,-19.96,;12.13,-19.19,;7.93,-13.46,;7.93,-11.92,;6.59,-14.23,;5.25,-13.45,;5.28,-11.91,;6.62,-11.16,;6.63,-9.62,;5.31,-8.84,;3.97,-9.59,;3.95,-11.12,;15.9,-10.33,;14.56,-9.57,;17.22,-9.55,;17.21,-8.01,;15.87,-7.26,;15.85,-5.72,;17.19,-4.93,;17.5,-3.42,;19.03,-3.25,;19.67,-4.66,;18.53,-5.69,;18.54,-7.23,;23.93,-13.34,;23.91,-11.8,;25.27,-14.1,;26.59,-13.31,;27.93,-14.07,;29.26,-13.29,;27.95,-15.61,)| Show InChI InChI=1S/C96H110N12O23S2/c97-93(125)76(18-10-11-40-98-96(132)103-65-25-30-72(75(46-65)95(127)128)92-73-31-28-70(109)47-78(73)131-79-48-71(110)29-32-74(79)92)104-84(114)56-107-51-68(42-60-14-6-2-7-15-60)101-83(113)54-105(86(116)35-38-91(123)124)49-66(26-36-89(119)120)99-82(112)55-108(88(118)45-63-21-33-77-80(44-63)130-58-129-77)50-67(27-37-90(121)122)100-81(111)53-106(85(115)34-22-59-12-4-1-5-13-59)52-69(43-61-16-8-3-9-17-61)102-94(126)64-23-19-62(20-24-64)57-133-41-39-87(107)117/h2-3,6-9,14-17,19-21,23-25,28-33,44,46-48,59,66-69,76,109H,1,4-5,10-13,18,22,26-27,34-43,45,49-58H2,(H2,97,125)(H,99,112)(H,100,111)(H,101,113)(H,102,126)(H,104,114)(H,119,120)(H,121,122)(H,123,124)(H,127,128)(H2,98,103,132)/t66-,67-,68-,69-,76-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human GST-tagged EphA2 cytoplasmic domain (560 to 976 residues) expressed in baculovirus expression system by fluores... |
J Med Chem 60: 9290-9298 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01280
BindingDB Entry DOI: 10.7270/Q20C4Z5R |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588605
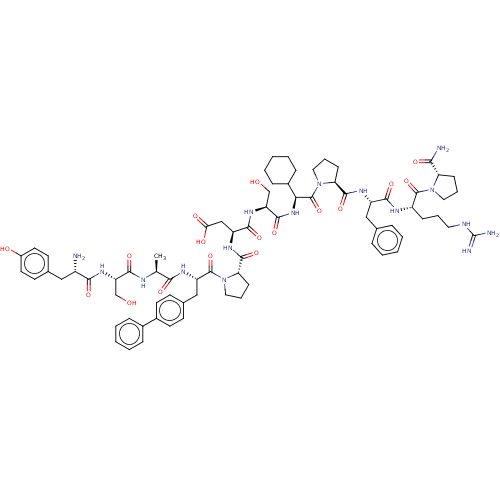
(CHEMBL5184907)Show SMILES C[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588594
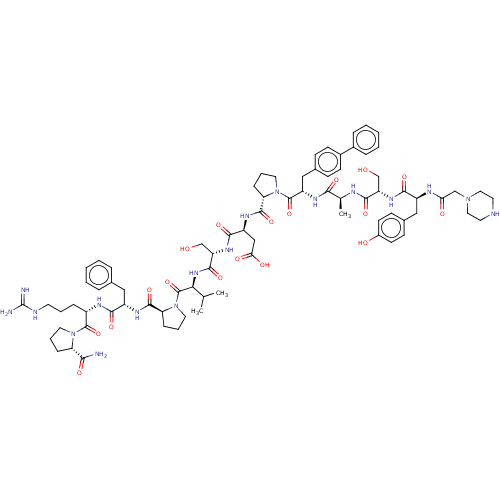
(CHEMBL5200977)Show SMILES CC(C)[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CN1CCNCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588586
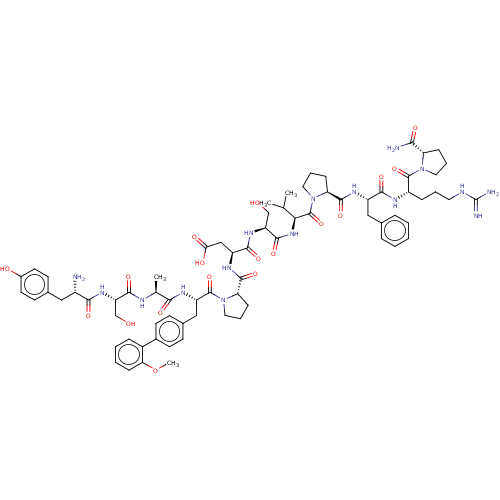
(CHEMBL5192927)Show SMILES COc1ccccc1-c1ccc(C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2CCC[C@H]2C(N)=O)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM50382959

(CEP-32496 | CHEMBL2029988 | US9730937, Example 261)Show SMILES COc1cc2ncnc(Oc3cccc(NC(=O)Nc4cc(on4)C(C)(C)C(F)(F)F)c3)c2cc1OC Show InChI InChI=1S/C24H22F3N5O5/c1-23(2,24(25,26)27)19-11-20(32-37-19)31-22(33)30-13-6-5-7-14(8-13)36-21-15-9-17(34-3)18(35-4)10-16(15)28-12-29-21/h5-12H,1-4H3,(H2,30,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Kinobeads competition assays were performed in 96-well format as previously described using mixed protein lysates of four cancer cell lines (K-562, M... |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50306682

((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1cnn(c1)C1CCNCC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C21H22Cl2FN5O/c1-12(19-16(22)2-3-17(24)20(19)23)30-18-8-13(9-27-21(18)25)14-10-28-29(11-14)15-4-6-26-7-5-15/h2-3,8-12,15,26H,4-7H2,1H3,(H2,25,27)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHA2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588585
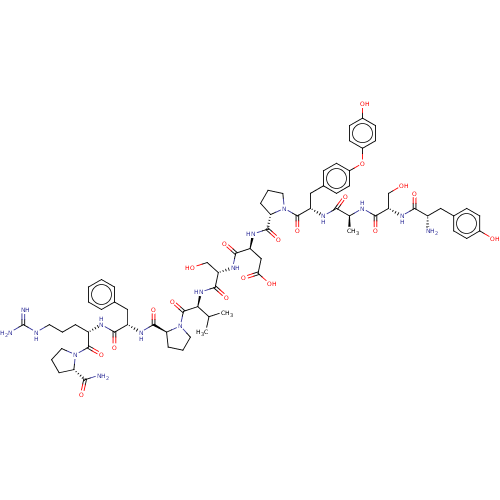
(CHEMBL5183595)Show SMILES CC(C)[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(Oc2ccc(O)cc2)cc1)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588593
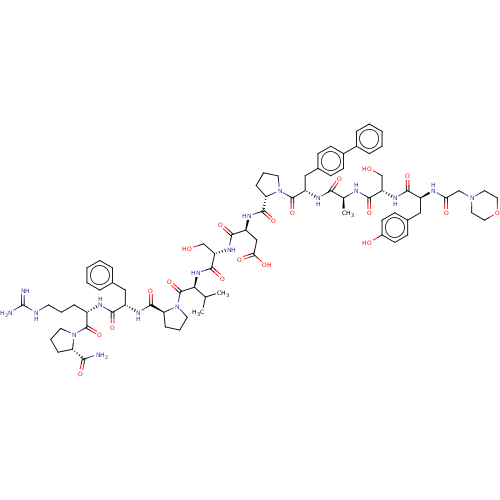
(CHEMBL5178709)Show SMILES CC(C)[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CN1CCOCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM60665
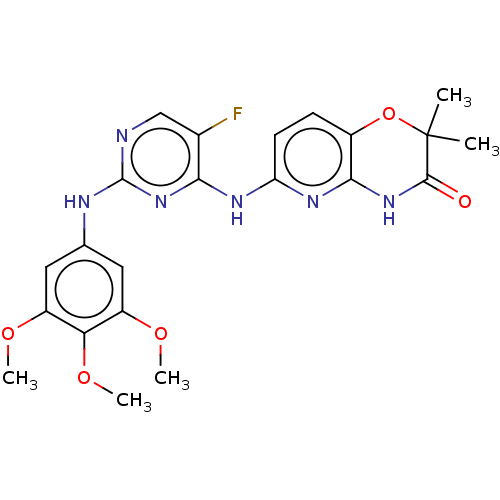
(BDBM50249542 | US9145414, R406 | US9212178, R406)Show SMILES COc1cc(Nc2ncc(F)c(Nc3ccc4OC(C)(C)C(=O)Nc4n3)n2)cc(OC)c1OC Show InChI InChI=1S/C22H23FN6O5/c1-22(2)20(30)28-19-13(34-22)6-7-16(27-19)26-18-12(23)10-24-21(29-18)25-11-8-14(31-3)17(33-5)15(9-11)32-4/h6-10H,1-5H3,(H3,24,25,26,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHA2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588598
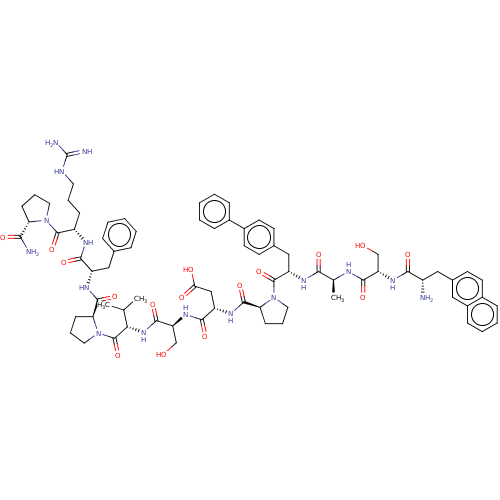
(CHEMBL5186123)Show SMILES CC(C)[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@@H](N)Cc1ccc2ccccc2c1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588589
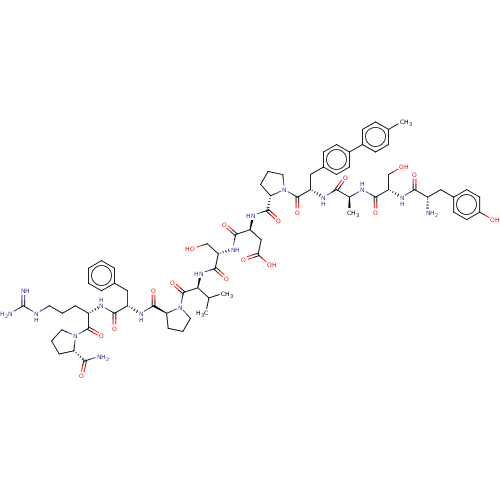
(CHEMBL5187055)Show SMILES CC(C)[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccc(C)cc1)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588587
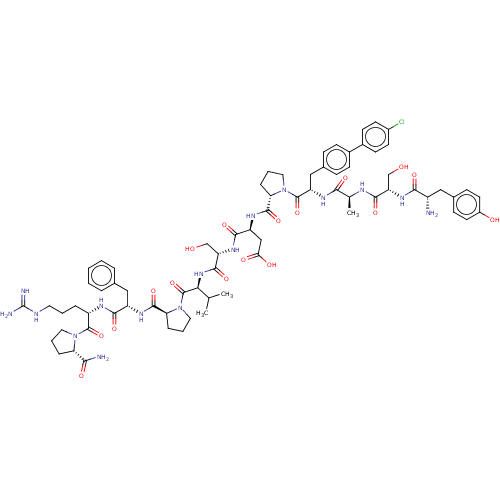
(CHEMBL5209493)Show SMILES CC(C)[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccc(Cl)cc1)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM31085
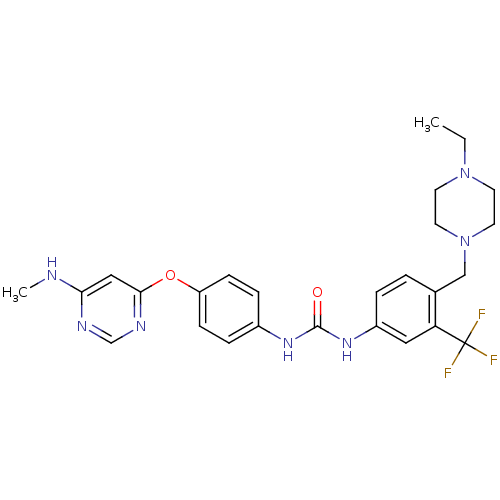
(1-[4-[(4-ethyl-1-piperazinyl)methyl]-3-(trifluorom...)Show SMILES CCN1CCN(Cc2ccc(NC(=O)Nc3ccc(Oc4cc(NC)ncn4)cc3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H30F3N7O2/c1-3-35-10-12-36(13-11-35)16-18-4-5-20(14-22(18)26(27,28)29)34-25(37)33-19-6-8-21(9-7-19)38-24-15-23(30-2)31-17-32-24/h4-9,14-15,17H,3,10-13,16H2,1-2H3,(H,30,31,32)(H2,33,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHA2 kinase domain |
Nat Biotechnol 26: 127-32 (2008)
Article DOI: 10.1038/nbt1358
BindingDB Entry DOI: 10.7270/Q2TT4RX2 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM31085

(1-[4-[(4-ethyl-1-piperazinyl)methyl]-3-(trifluorom...)Show SMILES CCN1CCN(Cc2ccc(NC(=O)Nc3ccc(Oc4cc(NC)ncn4)cc3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H30F3N7O2/c1-3-35-10-12-36(13-11-35)16-18-4-5-20(14-22(18)26(27,28)29)34-25(37)33-19-6-8-21(9-7-19)38-24-15-23(30-2)31-17-32-24/h4-9,14-15,17H,3,10-13,16H2,1-2H3,(H,30,31,32)(H2,33,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PCBioAssay
| n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2513WK1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM31085

(1-[4-[(4-ethyl-1-piperazinyl)methyl]-3-(trifluorom...)Show SMILES CCN1CCN(Cc2ccc(NC(=O)Nc3ccc(Oc4cc(NC)ncn4)cc3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H30F3N7O2/c1-3-35-10-12-36(13-11-35)16-18-4-5-20(14-22(18)26(27,28)29)34-25(37)33-19-6-8-21(9-7-19)38-24-15-23(30-2)31-17-32-24/h4-9,14-15,17H,3,10-13,16H2,1-2H3,(H,30,31,32)(H2,33,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHA2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM50311316
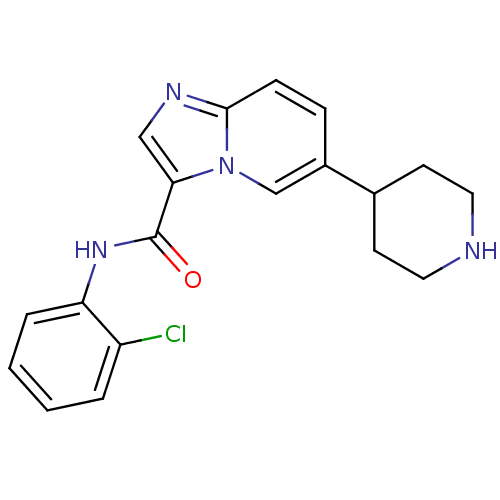
(CHEMBL1077739 | LDN-211904 | N-(2-chlorophenyl)-6-...)Show InChI InChI=1S/C19H19ClN4O/c20-15-3-1-2-4-16(15)23-19(25)17-11-22-18-6-5-14(12-24(17)18)13-7-9-21-10-8-13/h1-6,11-13,21H,7-10H2,(H,23,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Kinobeads competition assays were performed in 96-well format as previously described using mixed protein lysates of four cancer cell lines (K-562, M... |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588599
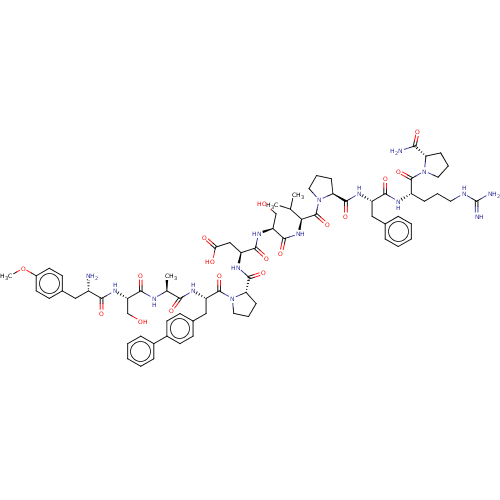
(CHEMBL5203013)Show SMILES COc1ccc(C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc2ccc(cc2)-c2ccccc2)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2CCC[C@H]2C(N)=O)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM50277545

(4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...)Show SMILES COc1cc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2OC)ccc1C(O)=O |c:11| Show InChI InChI=1S/C27H20ClFN4O4/c1-36-21-5-3-4-20(29)23(21)25-19-10-15(28)6-8-17(19)24-14(12-30-25)13-31-27(33-24)32-16-7-9-18(26(34)35)22(11-16)37-2/h3-11,13H,12H2,1-2H3,(H,34,35)(H,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 187 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Kinobeads competition assays were performed in 96-well format as previously described using mixed protein lysates of four cancer cell lines (K-562, M... |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588596

(CHEMBL5185590)Show SMILES CC(C)[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@@H](N)Cc1c[nH]c2cc(O)ccc12)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 187 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588601
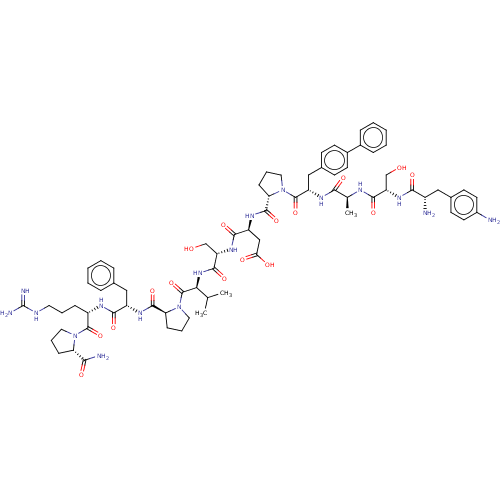
(CHEMBL5192872)Show SMILES CC(C)[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@@H](N)Cc1ccc(N)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM209860
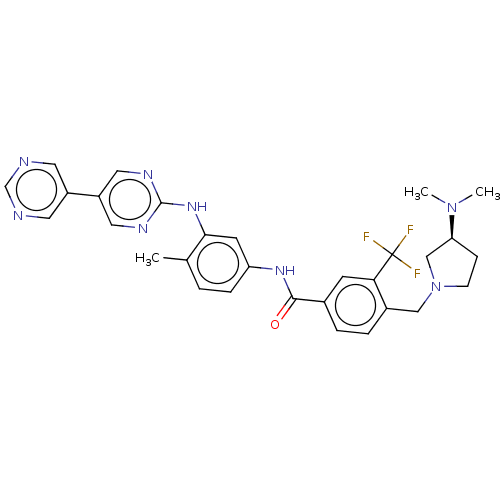
(4-[[(3S)-3-(dimethylamino)pyrrolidin-1-yl]methyl]-...)Show SMILES CN(C)[C@H]1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(Nc3ncc(cn3)-c3cncnc3)c2)C1 Show InChI InChI=1S/C30H31F3N8O/c1-19-4-7-24(11-27(19)39-29-36-14-23(15-37-29)22-12-34-18-35-13-22)38-28(42)20-5-6-21(26(10-20)30(31,32)33)16-41-9-8-25(17-41)40(2)3/h4-7,10-15,18,25H,8-9,16-17H2,1-3H3,(H,38,42)(H,36,37,39)/t25-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 192 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Kinobeads competition assays were performed in 96-well format as previously described using mixed protein lysates of four cancer cell lines (K-562, M... |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM209861
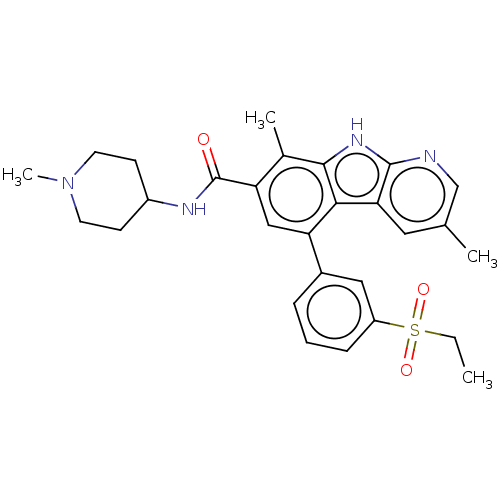
(5-(3-ethylsulfonylphenyl)-3,8-dimethyl-N-(1-methyl...)Show SMILES CCS(=O)(=O)c1cccc(c1)-c1cc(C(=O)NC2CCN(C)CC2)c(C)c2[nH]c3ncc(C)cc3c12 Show InChI InChI=1S/C28H32N4O3S/c1-5-36(34,35)21-8-6-7-19(14-21)23-15-22(28(33)30-20-9-11-32(4)12-10-20)18(3)26-25(23)24-13-17(2)16-29-27(24)31-26/h6-8,13-16,20H,5,9-12H2,1-4H3,(H,29,31)(H,30,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 198 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Kinobeads competition assays were performed in 96-well format as previously described using mixed protein lysates of four cancer cell lines (K-562, M... |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | |
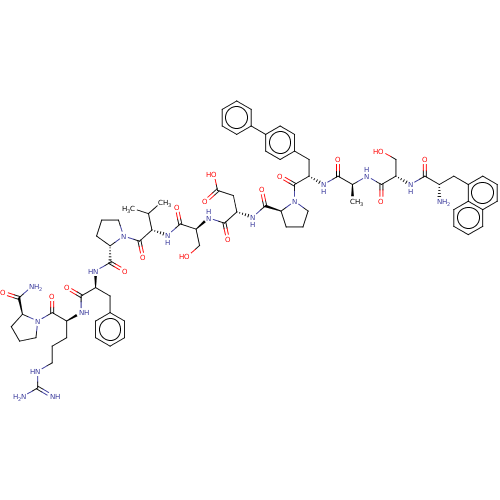
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588597
(CHEMBL5173083)Show SMILES CC(C)[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@@H](N)Cc1cccc2ccccc12)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 202 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588600
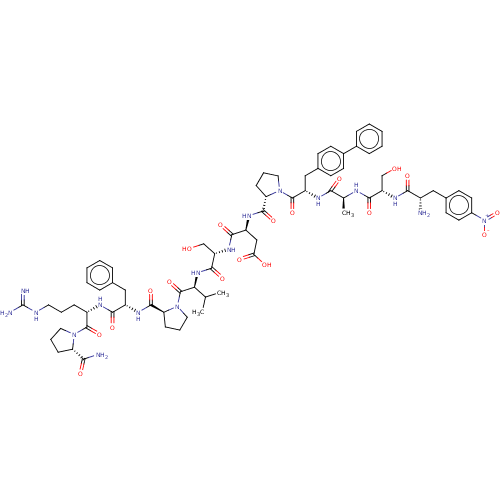
(CHEMBL5208655)Show SMILES CC(C)[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@@H](N)Cc1ccc(cc1)[N+]([O-])=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 204 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50237710
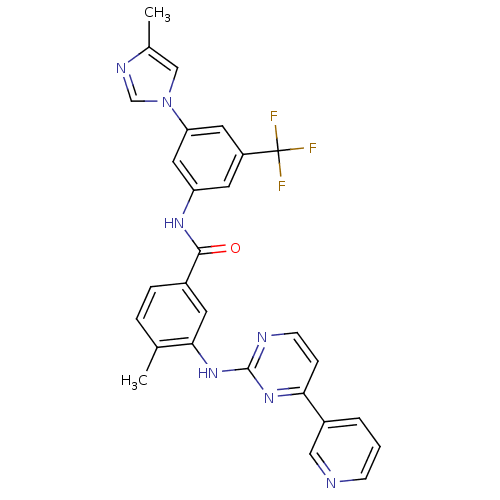
(4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifl...)Show SMILES Cc1cn(cn1)-c1cc(NC(=O)c2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)cc(c1)C(F)(F)F Show InChI InChI=1S/C28H22F3N7O/c1-17-5-6-19(10-25(17)37-27-33-9-7-24(36-27)20-4-3-8-32-14-20)26(39)35-22-11-21(28(29,30)31)12-23(13-22)38-15-18(2)34-16-38/h3-16H,1-2H3,(H,35,39)(H,33,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHA2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50588584
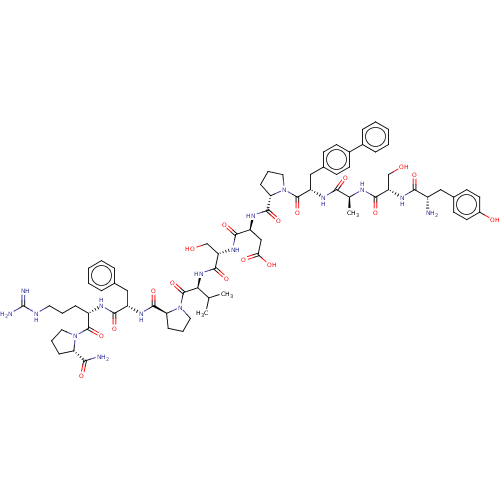
(CHEMBL5194630)Show SMILES CC(C)[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01391
BindingDB Entry DOI: 10.7270/Q2V4105C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data