 Found 55 hits of ic50 for UniProtKB: P54764
Found 55 hits of ic50 for UniProtKB: P54764 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphA4 by [gamma33-P]ATP based assay |
J Med Chem 52: 6433-46 (2009)
Article DOI: 10.1021/jm9009444
BindingDB Entry DOI: 10.7270/Q2BZ663G |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human EPHA4 using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.02.022
BindingDB Entry DOI: 10.7270/Q2DJ5KB8 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50157880
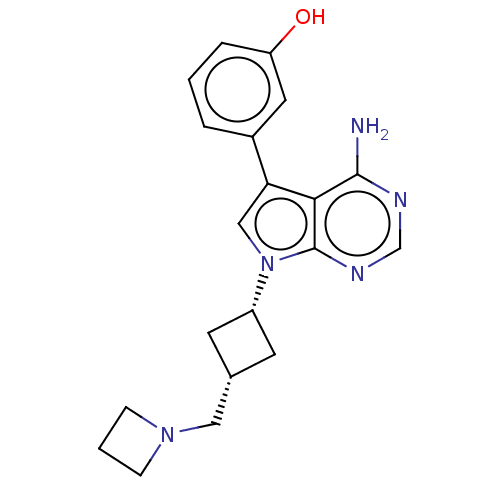
(CHEMBL3787112)Show SMILES Nc1ncnc2n(cc(-c3cccc(O)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:17.19,19.22,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;1.1,6.31,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C20H23N5O/c21-19-18-17(14-3-1-4-16(26)9-14)11-25(20(18)23-12-22-19)15-7-13(8-15)10-24-5-2-6-24/h1,3-4,9,11-13,15,26H,2,5-8,10H2,(H2,21,22,23)/t13-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EphA4 (unknown origin) in presence of [gamma33P]ATP |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50535199

(CHEMBL4462106)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCC(O)=O)NC(=O)CCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)N |r| Show InChI InChI=1S/C67H91N17O18S2/c1-34(2)55-65(101)80-47(29-37-14-18-40(86)19-15-37)60(96)76-43(10-6-25-73-67(70)71)57(93)72-26-24-52(87)75-44(20-22-53(88)89)58(94)78-48(30-38-31-74-42-9-5-4-8-41(38)42)62(98)77-45(21-23-54(90)91)59(95)81-49(56(69)92)32-103-104-33-50(63(99)83-55)82-61(97)46(28-36-12-16-39(85)17-13-36)79-64(100)51-11-7-27-84(51)66(102)35(3)68/h4-5,8-9,12-19,31,34-35,43-51,55,74,85-86H,6-7,10-11,20-30,32-33,68H2,1-3H3,(H2,69,92)(H,72,93)(H,75,87)(H,76,96)(H,77,98)(H,78,94)(H,79,100)(H,80,101)(H,81,95)(H,82,97)(H,83,99)(H,88,89)(H,90,91)(H4,70,71,73)/t35-,43-,44-,45-,46-,47-,48-,49-,50-,51-,55-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against human EphA4 receptor assessed as inhibition of binding of alkaline phosphatase-fused ephrin-A5 to immobilized EphA4 Fc by... |
ACS Med Chem Lett 7: 841-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00132
BindingDB Entry DOI: 10.7270/Q2TT4VF1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50535195
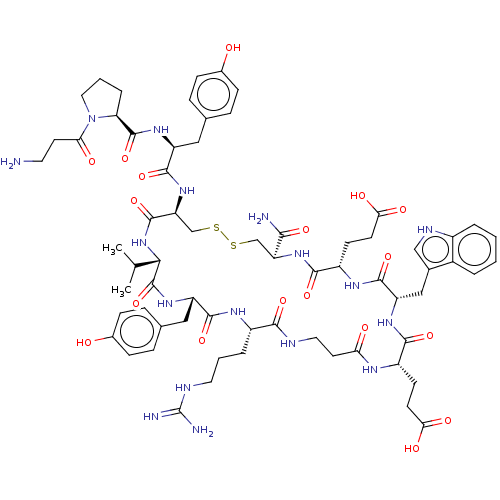
(CHEMBL4530593)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCC(O)=O)NC(=O)CCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)CCN |r| Show InChI InChI=1S/C67H91N17O18S2/c1-35(2)56-66(102)80-47(30-37-13-17-40(86)18-14-37)61(97)76-43(9-5-26-73-67(70)71)58(94)72-27-24-52(87)75-44(19-21-54(89)90)59(95)78-48(31-38-32-74-42-8-4-3-7-41(38)42)63(99)77-45(20-22-55(91)92)60(96)81-49(57(69)93)33-103-104-34-50(64(100)83-56)82-62(98)46(29-36-11-15-39(85)16-12-36)79-65(101)51-10-6-28-84(51)53(88)23-25-68/h3-4,7-8,11-18,32,35,43-51,56,74,85-86H,5-6,9-10,19-31,33-34,68H2,1-2H3,(H2,69,93)(H,72,94)(H,75,87)(H,76,97)(H,77,99)(H,78,95)(H,79,101)(H,80,102)(H,81,96)(H,82,98)(H,83,100)(H,89,90)(H,91,92)(H4,70,71,73)/t43-,44-,45-,46-,47-,48-,49-,50-,51-,56-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against human EphA4 receptor assessed as inhibition of binding of alkaline phosphatase-fused ephrin-A5 to immobilized EphA4 Fc by... |
ACS Med Chem Lett 7: 841-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00132
BindingDB Entry DOI: 10.7270/Q2TT4VF1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human EPHA4 using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay |
Eur J Med Chem 161: 456-467 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.052
BindingDB Entry DOI: 10.7270/Q2W380MT |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50535185
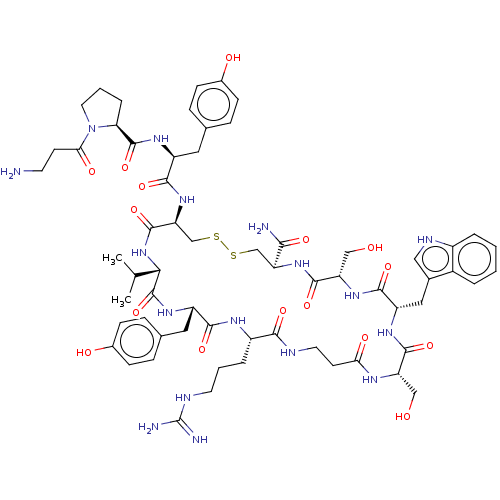
(CHEMBL4464968)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CO)NC(=O)CCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)CCN |r| Show InChI InChI=1S/C63H87N17O16S2/c1-33(2)52-62(96)75-43(26-35-13-17-38(84)18-14-35)55(89)72-41(9-5-22-69-63(66)67)54(88)68-23-20-50(85)71-45(29-81)58(92)73-44(27-36-28-70-40-8-4-3-7-39(36)40)57(91)76-46(30-82)59(93)77-47(53(65)87)31-97-98-32-48(60(94)79-52)78-56(90)42(25-34-11-15-37(83)16-12-34)74-61(95)49-10-6-24-80(49)51(86)19-21-64/h3-4,7-8,11-18,28,33,41-49,52,70,81-84H,5-6,9-10,19-27,29-32,64H2,1-2H3,(H2,65,87)(H,68,88)(H,71,85)(H,72,89)(H,73,92)(H,74,95)(H,75,96)(H,76,91)(H,77,93)(H,78,90)(H,79,94)(H4,66,67,69)/t41-,42-,43-,44-,45-,46-,47-,48-,49-,52-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against human EphA4 receptor assessed as inhibition of binding of alkaline phosphatase-fused ephrin-A5 to immobilized EphA4 Fc by... |
ACS Med Chem Lett 7: 841-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00132
BindingDB Entry DOI: 10.7270/Q2TT4VF1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50535194
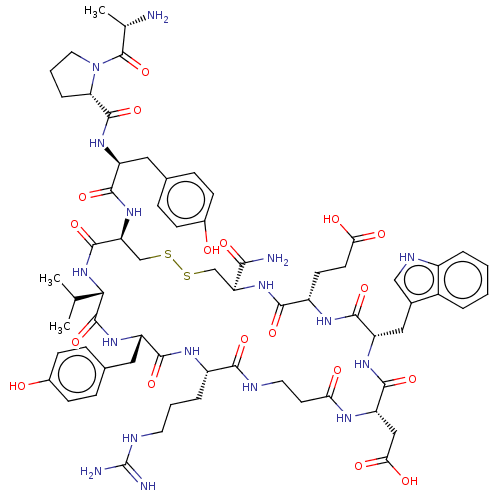
(CHEMBL4464855)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CC(O)=O)NC(=O)CCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)N |r| Show InChI InChI=1S/C66H89N17O18S2/c1-33(2)54-64(100)79-45(27-36-14-18-39(85)19-15-36)58(94)75-42(10-6-23-72-66(69)70)56(92)71-24-22-51(86)74-47(29-53(89)90)61(97)77-46(28-37-30-73-41-9-5-4-8-40(37)41)60(96)76-43(20-21-52(87)88)57(93)80-48(55(68)91)31-102-103-32-49(62(98)82-54)81-59(95)44(26-35-12-16-38(84)17-13-35)78-63(99)50-11-7-25-83(50)65(101)34(3)67/h4-5,8-9,12-19,30,33-34,42-50,54,73,84-85H,6-7,10-11,20-29,31-32,67H2,1-3H3,(H2,68,91)(H,71,92)(H,74,86)(H,75,94)(H,76,96)(H,77,97)(H,78,99)(H,79,100)(H,80,93)(H,81,95)(H,82,98)(H,87,88)(H,89,90)(H4,69,70,72)/t34-,42-,43-,44-,45-,46-,47-,48-,49-,50-,54-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against human EphA4 receptor assessed as inhibition of binding of alkaline phosphatase-fused ephrin-A5 to immobilized EphA4 Fc by... |
ACS Med Chem Lett 7: 841-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00132
BindingDB Entry DOI: 10.7270/Q2TT4VF1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50535198
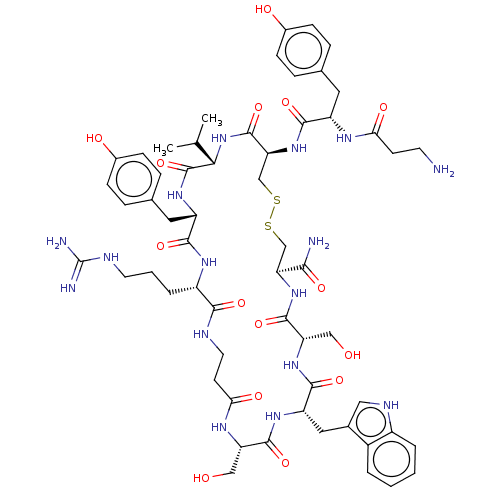
(CHEMBL4440005)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CO)NC(=O)CCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCN |r| Show InChI InChI=1S/C58H80N16O15S2/c1-30(2)48-57(89)70-40(23-32-11-15-35(78)16-12-32)52(84)68-38(8-5-20-64-58(61)62)50(82)63-21-18-47(80)67-42(26-75)54(86)69-41(24-33-25-65-37-7-4-3-6-36(33)37)53(85)71-43(27-76)55(87)72-44(49(60)81)28-90-91-29-45(56(88)74-48)73-51(83)39(66-46(79)17-19-59)22-31-9-13-34(77)14-10-31/h3-4,6-7,9-16,25,30,38-45,48,65,75-78H,5,8,17-24,26-29,59H2,1-2H3,(H2,60,81)(H,63,82)(H,66,79)(H,67,80)(H,68,84)(H,69,86)(H,70,89)(H,71,85)(H,72,87)(H,73,83)(H,74,88)(H4,61,62,64)/t38-,39-,40-,41-,42-,43-,44-,45-,48-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against human EphA4 receptor assessed as inhibition of binding of alkaline phosphatase-fused ephrin-A5 to immobilized EphA4 Fc by... |
ACS Med Chem Lett 7: 841-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00132
BindingDB Entry DOI: 10.7270/Q2TT4VF1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50535186
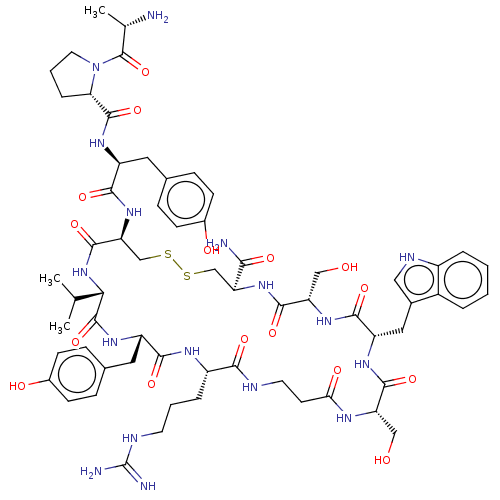
(CHEMBL4436732)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CO)NC(=O)CCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)N |r| Show InChI InChI=1S/C63H87N17O16S2/c1-32(2)51-61(95)75-43(25-35-14-18-38(84)19-15-35)54(88)72-41(10-6-21-69-63(66)67)53(87)68-22-20-50(85)71-45(28-81)57(91)73-44(26-36-27-70-40-9-5-4-8-39(36)40)56(90)76-46(29-82)58(92)77-47(52(65)86)30-97-98-31-48(59(93)79-51)78-55(89)42(24-34-12-16-37(83)17-13-34)74-60(94)49-11-7-23-80(49)62(96)33(3)64/h4-5,8-9,12-19,27,32-33,41-49,51,70,81-84H,6-7,10-11,20-26,28-31,64H2,1-3H3,(H2,65,86)(H,68,87)(H,71,85)(H,72,88)(H,73,91)(H,74,94)(H,75,95)(H,76,90)(H,77,92)(H,78,89)(H,79,93)(H4,66,67,69)/t33-,41-,42-,43-,44-,45-,46-,47-,48-,49-,51-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against human EphA4 receptor assessed as inhibition of binding of alkaline phosphatase-fused ephrin-A5 to immobilized EphA4 Fc by... |
ACS Med Chem Lett 7: 841-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00132
BindingDB Entry DOI: 10.7270/Q2TT4VF1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50535192
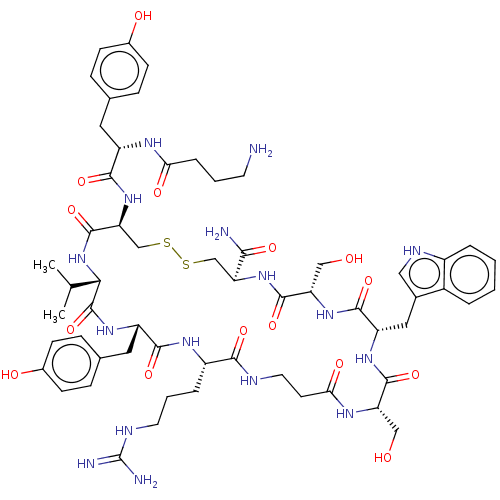
(CHEMBL4458170)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CO)NC(=O)CCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCCN |r| Show InChI InChI=1S/C59H82N16O15S2/c1-31(2)49-58(90)71-41(24-33-13-17-36(79)18-14-33)53(85)69-39(9-6-21-65-59(62)63)51(83)64-22-19-48(81)68-43(27-76)55(87)70-42(25-34-26-66-38-8-4-3-7-37(34)38)54(86)72-44(28-77)56(88)73-45(50(61)82)29-91-92-30-46(57(89)75-49)74-52(84)40(67-47(80)10-5-20-60)23-32-11-15-35(78)16-12-32/h3-4,7-8,11-18,26,31,39-46,49,66,76-79H,5-6,9-10,19-25,27-30,60H2,1-2H3,(H2,61,82)(H,64,83)(H,67,80)(H,68,81)(H,69,85)(H,70,87)(H,71,90)(H,72,86)(H,73,88)(H,74,84)(H,75,89)(H4,62,63,65)/t39-,40-,41-,42-,43-,44-,45-,46-,49-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against human EphA4 receptor assessed as inhibition of binding of alkaline phosphatase-fused ephrin-A5 to immobilized EphA4 Fc by... |
ACS Med Chem Lett 7: 841-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00132
BindingDB Entry DOI: 10.7270/Q2TT4VF1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50535196
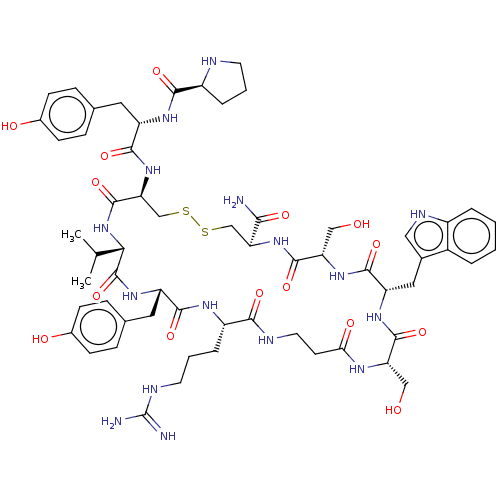
(CHEMBL4447628)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CO)NC(=O)CCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1 |r| Show InChI InChI=1S/C60H82N16O15S2/c1-31(2)49-59(91)72-42(24-33-13-17-36(80)18-14-33)53(85)69-40(10-6-21-66-60(62)63)51(83)65-22-19-48(81)68-44(27-77)56(88)71-43(25-34-26-67-38-8-4-3-7-37(34)38)55(87)73-45(28-78)57(89)74-46(50(61)82)29-92-93-30-47(58(90)76-49)75-54(86)41(23-32-11-15-35(79)16-12-32)70-52(84)39-9-5-20-64-39/h3-4,7-8,11-18,26,31,39-47,49,64,67,77-80H,5-6,9-10,19-25,27-30H2,1-2H3,(H2,61,82)(H,65,83)(H,68,81)(H,69,85)(H,70,84)(H,71,88)(H,72,91)(H,73,87)(H,74,89)(H,75,86)(H,76,90)(H4,62,63,66)/t39-,40-,41-,42-,43-,44-,45-,46-,47-,49-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against human EphA4 receptor assessed as inhibition of binding of alkaline phosphatase-fused ephrin-A5 to immobilized EphA4 Fc by... |
ACS Med Chem Lett 7: 841-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00132
BindingDB Entry DOI: 10.7270/Q2TT4VF1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50535193
(CHEMBL4539149)Show SMILES CNCC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CO)NC(=O)CCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](NC1=O)C(C)C)C(N)=O |r| Show InChI InChI=1S/C58H80N16O15S2/c1-30(2)48-57(89)70-40(22-32-12-16-35(78)17-13-32)52(84)68-38(9-6-19-64-58(60)61)50(82)63-20-18-46(79)67-42(26-75)54(86)69-41(23-33-24-65-37-8-5-4-7-36(33)37)53(85)71-43(27-76)55(87)72-44(49(59)81)28-90-91-29-45(56(88)74-48)73-51(83)39(66-47(80)25-62-3)21-31-10-14-34(77)15-11-31/h4-5,7-8,10-17,24,30,38-45,48,62,65,75-78H,6,9,18-23,25-29H2,1-3H3,(H2,59,81)(H,63,82)(H,66,80)(H,67,79)(H,68,84)(H,69,86)(H,70,89)(H,71,85)(H,72,87)(H,73,83)(H,74,88)(H4,60,61,64)/t38-,39-,40-,41-,42-,43-,44-,45-,48-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against human EphA4 receptor assessed as inhibition of binding of alkaline phosphatase-fused ephrin-A5 to immobilized EphA4 Fc by... |
ACS Med Chem Lett 7: 841-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00132
BindingDB Entry DOI: 10.7270/Q2TT4VF1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50535184
(CHEMBL4516686)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CO)NC(=O)CCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CN |r| Show InChI InChI=1S/C57H78N16O15S2/c1-29(2)47-56(88)69-39(21-31-11-15-34(77)16-12-31)51(83)67-37(8-5-18-63-57(60)61)49(81)62-19-17-45(78)66-41(25-74)53(85)68-40(22-32-24-64-36-7-4-3-6-35(32)36)52(84)70-42(26-75)54(86)71-43(48(59)80)27-89-90-28-44(55(87)73-47)72-50(82)38(65-46(79)23-58)20-30-9-13-33(76)14-10-30/h3-4,6-7,9-16,24,29,37-44,47,64,74-77H,5,8,17-23,25-28,58H2,1-2H3,(H2,59,80)(H,62,81)(H,65,79)(H,66,78)(H,67,83)(H,68,85)(H,69,88)(H,70,84)(H,71,86)(H,72,82)(H,73,87)(H4,60,61,63)/t37-,38-,39-,40-,41-,42-,43-,44-,47-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against human EphA4 receptor assessed as inhibition of binding of alkaline phosphatase-fused ephrin-A5 to immobilized EphA4 Fc by... |
ACS Med Chem Lett 7: 841-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00132
BindingDB Entry DOI: 10.7270/Q2TT4VF1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50535182

(CHEMBL4449863)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CO)NC(=O)CCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](C)N |r| Show InChI InChI=1S/C63H87N17O16S2/c1-32(2)51-61(95)75-43(25-35-14-18-38(84)19-15-35)54(88)72-41(10-6-21-69-63(66)67)53(87)68-22-20-50(85)71-45(28-81)57(91)73-44(26-36-27-70-40-9-5-4-8-39(36)40)56(90)76-46(29-82)58(92)77-47(52(65)86)30-97-98-31-48(59(93)79-51)78-55(89)42(24-34-12-16-37(83)17-13-34)74-60(94)49-11-7-23-80(49)62(96)33(3)64/h4-5,8-9,12-19,27,32-33,41-49,51,70,81-84H,6-7,10-11,20-26,28-31,64H2,1-3H3,(H2,65,86)(H,68,87)(H,71,85)(H,72,88)(H,73,91)(H,74,94)(H,75,95)(H,76,90)(H,77,92)(H,78,89)(H,79,93)(H4,66,67,69)/t33-,41+,42+,43+,44+,45+,46+,47+,48+,49+,51+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against human EphA4 receptor assessed as inhibition of binding of alkaline phosphatase-fused ephrin-A5 to immobilized EphA4 Fc by... |
ACS Med Chem Lett 7: 841-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00132
BindingDB Entry DOI: 10.7270/Q2TT4VF1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50535200
(CHEMBL4434643)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CO)NC(=O)CCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCCCN |r| Show InChI InChI=1S/C60H84N16O15S2/c1-32(2)50-59(91)72-42(25-34-14-18-37(80)19-15-34)54(86)70-40(10-7-22-66-60(63)64)52(84)65-23-20-49(82)69-44(28-77)56(88)71-43(26-35-27-67-39-9-4-3-8-38(35)39)55(87)73-45(29-78)57(89)74-46(51(62)83)30-92-93-31-47(58(90)76-50)75-53(85)41(68-48(81)11-5-6-21-61)24-33-12-16-36(79)17-13-33/h3-4,8-9,12-19,27,32,40-47,50,67,77-80H,5-7,10-11,20-26,28-31,61H2,1-2H3,(H2,62,83)(H,65,84)(H,68,81)(H,69,82)(H,70,86)(H,71,88)(H,72,91)(H,73,87)(H,74,89)(H,75,85)(H,76,90)(H4,63,64,66)/t40-,41-,42-,43-,44-,45-,46-,47-,50-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against human EphA4 receptor assessed as inhibition of binding of alkaline phosphatase-fused ephrin-A5 to immobilized EphA4 Fc by... |
ACS Med Chem Lett 7: 841-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00132
BindingDB Entry DOI: 10.7270/Q2TT4VF1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of EPHA4 |
Bioorg Med Chem Lett 21: 7155-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.078
BindingDB Entry DOI: 10.7270/Q2NC61NH |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50452149
(CHEMBL4216073)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cccc(c2)C(F)(F)F)ccc1C Show InChI InChI=1S/C24H19F3N4O2/c1-14-6-8-19(29-23(33)16-4-3-5-18(10-16)24(25,26)27)11-20(14)17-7-9-22-30-21(28-15(2)32)13-31(22)12-17/h3-13H,1-2H3,(H,28,32)(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EphA4 (unknown origin) |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50535181
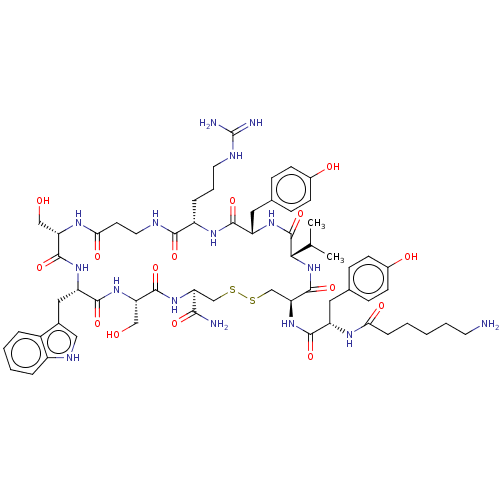
(CHEMBL4436577)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CO)NC(=O)CCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCCCCN |r| Show InChI InChI=1S/C61H86N16O15S2/c1-33(2)51-60(92)73-43(26-35-15-19-38(81)20-16-35)55(87)71-41(11-8-23-67-61(64)65)53(85)66-24-21-50(83)70-45(29-78)57(89)72-44(27-36-28-68-40-10-6-5-9-39(36)40)56(88)74-46(30-79)58(90)75-47(52(63)84)31-93-94-32-48(59(91)77-51)76-54(86)42(25-34-13-17-37(80)18-14-34)69-49(82)12-4-3-7-22-62/h5-6,9-10,13-20,28,33,41-48,51,68,78-81H,3-4,7-8,11-12,21-27,29-32,62H2,1-2H3,(H2,63,84)(H,66,85)(H,69,82)(H,70,83)(H,71,87)(H,72,89)(H,73,92)(H,74,88)(H,75,90)(H,76,86)(H,77,91)(H4,64,65,67)/t41-,42-,43-,44-,45-,46-,47-,48-,51-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against human EphA4 receptor assessed as inhibition of binding of alkaline phosphatase-fused ephrin-A5 to immobilized EphA4 Fc by... |
ACS Med Chem Lett 7: 841-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00132
BindingDB Entry DOI: 10.7270/Q2TT4VF1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 227 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant EPHA4 (601 to 892 residues) using poly(Glu,Tyr)4:1 as substrate incubated for 40 mins in presence of [gamma33P-ATP] b... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50535197
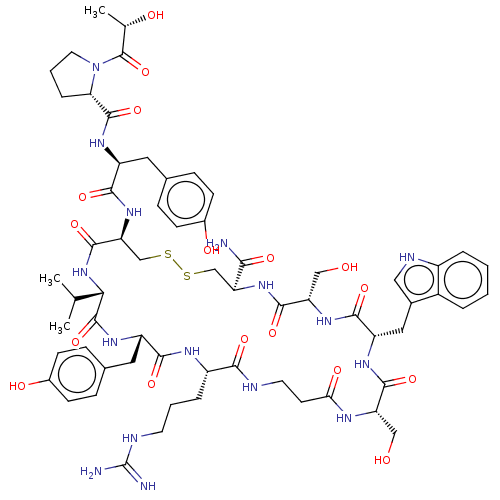
(CHEMBL4474623)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CO)NC(=O)CCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)O |r| Show InChI InChI=1S/C63H86N16O17S2/c1-32(2)51-61(95)74-43(25-35-14-18-38(84)19-15-35)54(88)71-41(10-6-21-68-63(65)66)53(87)67-22-20-50(85)70-45(28-80)57(91)72-44(26-36-27-69-40-9-5-4-8-39(36)40)56(90)75-46(29-81)58(92)76-47(52(64)86)30-97-98-31-48(59(93)78-51)77-55(89)42(24-34-12-16-37(83)17-13-34)73-60(94)49-11-7-23-79(49)62(96)33(3)82/h4-5,8-9,12-19,27,32-33,41-49,51,69,80-84H,6-7,10-11,20-26,28-31H2,1-3H3,(H2,64,86)(H,67,87)(H,70,85)(H,71,88)(H,72,91)(H,73,94)(H,74,95)(H,75,90)(H,76,92)(H,77,89)(H,78,93)(H4,65,66,68)/t33-,41-,42-,43-,44-,45-,46-,47-,48-,49-,51-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against human EphA4 receptor assessed as inhibition of binding of alkaline phosphatase-fused ephrin-A5 to immobilized EphA4 Fc by... |
ACS Med Chem Lett 7: 841-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00132
BindingDB Entry DOI: 10.7270/Q2TT4VF1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50535185

(CHEMBL4464968)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CO)NC(=O)CCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)CCN |r| Show InChI InChI=1S/C63H87N17O16S2/c1-33(2)52-62(96)75-43(26-35-13-17-38(84)18-14-35)55(89)72-41(9-5-22-69-63(66)67)54(88)68-23-20-50(85)71-45(29-81)58(92)73-44(27-36-28-70-40-8-4-3-7-39(36)40)57(91)76-46(30-82)59(93)77-47(53(65)87)31-97-98-32-48(60(94)79-52)78-56(90)42(25-34-11-15-37(83)16-12-34)74-61(95)49-10-6-24-80(49)51(86)19-21-64/h3-4,7-8,11-18,28,33,41-49,52,70,81-84H,5-6,9-10,19-27,29-32,64H2,1-2H3,(H2,65,87)(H,68,88)(H,71,85)(H,72,89)(H,73,92)(H,74,95)(H,75,96)(H,76,91)(H,77,93)(H,78,90)(H,79,94)(H4,66,67,69)/t41-,42-,43-,44-,45-,46-,47-,48-,49-,52-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 0.5 ug/ml ephrinA5-induced human EphA4 tyrosine phosphorylation expressed in HEK293AD cells preincubated for 20 mins followed by ephrin... |
ACS Med Chem Lett 7: 841-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00132
BindingDB Entry DOI: 10.7270/Q2TT4VF1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50535190
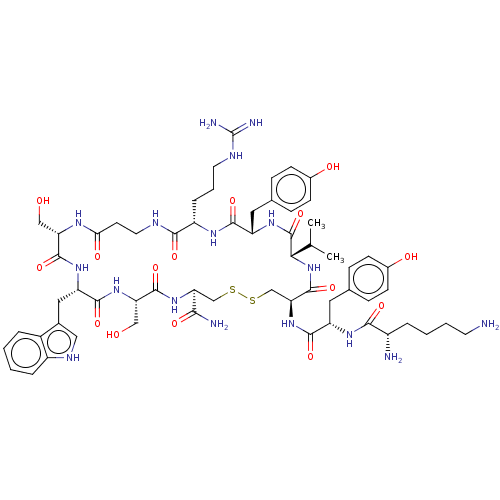
(CHEMBL4520376)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CO)NC(=O)CCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)CCCCN |r| Show InChI InChI=1S/C61H87N17O15S2/c1-32(2)50-60(93)74-43(25-34-14-18-37(82)19-15-34)54(87)71-41(11-7-22-68-61(65)66)53(86)67-23-20-49(83)70-45(28-79)57(90)73-44(26-35-27-69-40-10-4-3-8-38(35)40)56(89)75-46(29-80)58(91)76-47(51(64)84)30-94-95-31-48(59(92)78-50)77-55(88)42(24-33-12-16-36(81)17-13-33)72-52(85)39(63)9-5-6-21-62/h3-4,8,10,12-19,27,32,39,41-48,50,69,79-82H,5-7,9,11,20-26,28-31,62-63H2,1-2H3,(H2,64,84)(H,67,86)(H,70,83)(H,71,87)(H,72,85)(H,73,90)(H,74,93)(H,75,89)(H,76,91)(H,77,88)(H,78,92)(H4,65,66,68)/t39-,41-,42-,43-,44-,45-,46-,47-,48-,50-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against human EphA4 receptor assessed as inhibition of binding of alkaline phosphatase-fused ephrin-A5 to immobilized EphA4 Fc by... |
ACS Med Chem Lett 7: 841-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00132
BindingDB Entry DOI: 10.7270/Q2TT4VF1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50535183
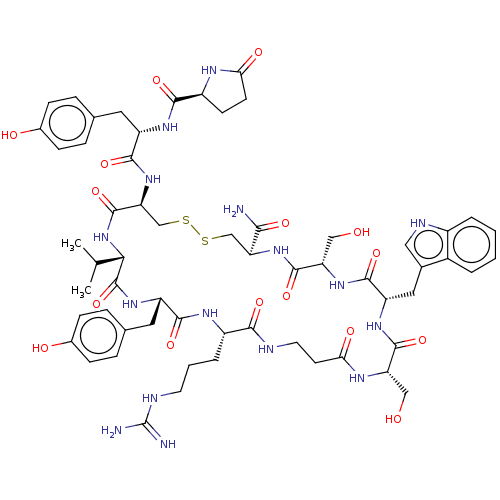
(CHEMBL4569881)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CO)NC(=O)CCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCC(=O)N1 |r| Show InChI InChI=1S/C60H80N16O16S2/c1-30(2)49-59(92)72-41(23-32-11-15-35(80)16-12-32)53(86)69-38(8-5-20-65-60(62)63)51(84)64-21-19-48(82)68-43(26-77)56(89)71-42(24-33-25-66-37-7-4-3-6-36(33)37)55(88)73-44(27-78)57(90)74-45(50(61)83)28-93-94-29-46(58(91)76-49)75-54(87)40(22-31-9-13-34(79)14-10-31)70-52(85)39-17-18-47(81)67-39/h3-4,6-7,9-16,25,30,38-46,49,66,77-80H,5,8,17-24,26-29H2,1-2H3,(H2,61,83)(H,64,84)(H,67,81)(H,68,82)(H,69,86)(H,70,85)(H,71,89)(H,72,92)(H,73,88)(H,74,90)(H,75,87)(H,76,91)(H4,62,63,65)/t38-,39-,40-,41-,42-,43-,44-,45-,46-,49-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against human EphA4 receptor assessed as inhibition of binding of alkaline phosphatase-fused ephrin-A5 to immobilized EphA4 Fc by... |
ACS Med Chem Lett 7: 841-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00132
BindingDB Entry DOI: 10.7270/Q2TT4VF1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50535195

(CHEMBL4530593)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCC(O)=O)NC(=O)CCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)CCN |r| Show InChI InChI=1S/C67H91N17O18S2/c1-35(2)56-66(102)80-47(30-37-13-17-40(86)18-14-37)61(97)76-43(9-5-26-73-67(70)71)58(94)72-27-24-52(87)75-44(19-21-54(89)90)59(95)78-48(31-38-32-74-42-8-4-3-7-41(38)42)63(99)77-45(20-22-55(91)92)60(96)81-49(57(69)93)33-103-104-34-50(64(100)83-56)82-62(98)46(29-36-11-15-39(85)16-12-36)79-65(101)51-10-6-28-84(51)53(88)23-25-68/h3-4,7-8,11-18,32,35,43-51,56,74,85-86H,5-6,9-10,19-31,33-34,68H2,1-2H3,(H2,69,93)(H,72,94)(H,75,87)(H,76,97)(H,77,99)(H,78,95)(H,79,101)(H,80,102)(H,81,96)(H,82,98)(H,83,100)(H,89,90)(H,91,92)(H4,70,71,73)/t43-,44-,45-,46-,47-,48-,49-,50-,51-,56-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 0.4 ug/ml ephrinA5-induced human EphA4 tyrosine phosphorylation expressed in HEK293AD cells preincubated for 20 mins followed by ephrin... |
ACS Med Chem Lett 7: 841-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00132
BindingDB Entry DOI: 10.7270/Q2TT4VF1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50535189
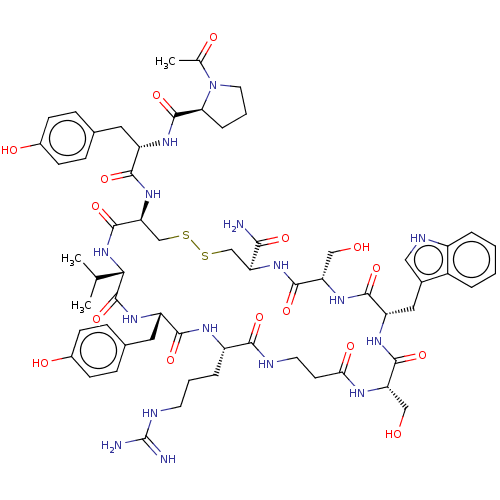
(CHEMBL4442705)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CO)NC(=O)CCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(C)=O |r| Show InChI InChI=1S/C62H84N16O16S2/c1-32(2)51-61(94)73-43(25-35-14-18-38(83)19-15-35)54(87)70-41(10-6-21-67-62(64)65)53(86)66-22-20-50(84)69-45(28-79)57(90)71-44(26-36-27-68-40-9-5-4-8-39(36)40)56(89)74-46(29-80)58(91)75-47(52(63)85)30-95-96-31-48(59(92)77-51)76-55(88)42(24-34-12-16-37(82)17-13-34)72-60(93)49-11-7-23-78(49)33(3)81/h4-5,8-9,12-19,27,32,41-49,51,68,79-80,82-83H,6-7,10-11,20-26,28-31H2,1-3H3,(H2,63,85)(H,66,86)(H,69,84)(H,70,87)(H,71,90)(H,72,93)(H,73,94)(H,74,89)(H,75,91)(H,76,88)(H,77,92)(H4,64,65,67)/t41-,42-,43-,44-,45-,46-,47-,48-,49-,51-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against human EphA4 receptor assessed as inhibition of binding of alkaline phosphatase-fused ephrin-A5 to immobilized EphA4 Fc by... |
ACS Med Chem Lett 7: 841-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00132
BindingDB Entry DOI: 10.7270/Q2TT4VF1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50535191
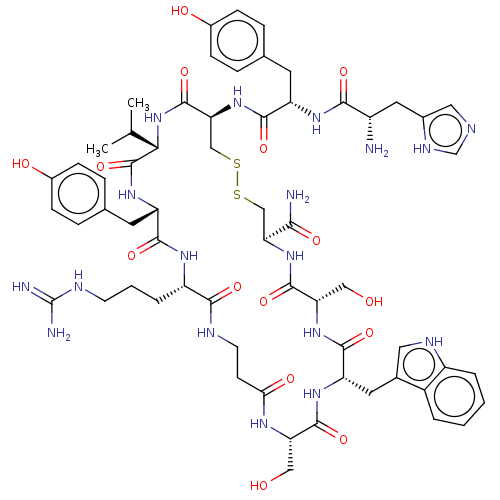
(CHEMBL4522324)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CO)NC(=O)CCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)Cc1cnc[nH]1 |r| Show InChI InChI=1S/C61H82N18O15S2/c1-31(2)50-60(94)75-43(21-33-11-15-37(83)16-12-33)54(88)72-41(8-5-18-68-61(64)65)53(87)67-19-17-49(84)71-45(26-80)57(91)74-44(22-34-24-69-40-7-4-3-6-38(34)40)56(90)76-46(27-81)58(92)77-47(51(63)85)28-95-96-29-48(59(93)79-50)78-55(89)42(20-32-9-13-36(82)14-10-32)73-52(86)39(62)23-35-25-66-30-70-35/h3-4,6-7,9-16,24-25,30-31,39,41-48,50,69,80-83H,5,8,17-23,26-29,62H2,1-2H3,(H2,63,85)(H,66,70)(H,67,87)(H,71,84)(H,72,88)(H,73,86)(H,74,91)(H,75,94)(H,76,90)(H,77,92)(H,78,89)(H,79,93)(H4,64,65,68)/t39-,41-,42-,43-,44-,45-,46-,47-,48-,50-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against human EphA4 receptor assessed as inhibition of binding of alkaline phosphatase-fused ephrin-A5 to immobilized EphA4 Fc by... |
ACS Med Chem Lett 7: 841-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00132
BindingDB Entry DOI: 10.7270/Q2TT4VF1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50535188
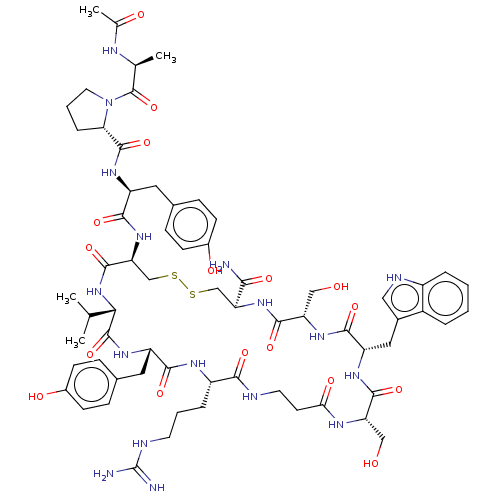
(CHEMBL4591478)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CO)NC(=O)CCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(C)=O |r| Show InChI InChI=1S/C65H89N17O17S2/c1-33(2)53-63(98)77-45(26-37-15-19-40(87)20-16-37)56(91)74-43(11-7-22-70-65(67)68)55(90)69-23-21-52(88)73-47(29-83)59(94)75-46(27-38-28-71-42-10-6-5-9-41(38)42)58(93)78-48(30-84)60(95)79-49(54(66)89)31-100-101-32-50(61(96)81-53)80-57(92)44(25-36-13-17-39(86)18-14-36)76-62(97)51-12-8-24-82(51)64(99)34(3)72-35(4)85/h5-6,9-10,13-20,28,33-34,43-51,53,71,83-84,86-87H,7-8,11-12,21-27,29-32H2,1-4H3,(H2,66,89)(H,69,90)(H,72,85)(H,73,88)(H,74,91)(H,75,94)(H,76,97)(H,77,98)(H,78,93)(H,79,95)(H,80,92)(H,81,96)(H4,67,68,70)/t34-,43-,44-,45-,46-,47-,48-,49-,50-,51-,53-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against human EphA4 receptor assessed as inhibition of binding of alkaline phosphatase-fused ephrin-A5 to immobilized EphA4 Fc by... |
ACS Med Chem Lett 7: 841-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00132
BindingDB Entry DOI: 10.7270/Q2TT4VF1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50535187

(CHEMBL4476251)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CO)NC(=O)CCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(N)=O)NC(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C55H75N15O14S2/c1-28(2)45-54(84)66-38(21-30-11-15-33(74)16-12-30)49(79)64-37(8-5-18-61-55(58)59)48(78)60-19-17-44(75)63-40(24-71)51(81)65-39(22-31-23-62-36-7-4-3-6-34(31)36)50(80)67-41(25-72)52(82)68-42(46(57)76)26-85-86-27-43(53(83)70-45)69-47(77)35(56)20-29-9-13-32(73)14-10-29/h3-4,6-7,9-16,23,28,35,37-43,45,62,71-74H,5,8,17-22,24-27,56H2,1-2H3,(H2,57,76)(H,60,78)(H,63,75)(H,64,79)(H,65,81)(H,66,84)(H,67,80)(H,68,82)(H,69,77)(H,70,83)(H4,58,59,61)/t35-,37-,38-,39-,40-,41-,42-,43-,45-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against human EphA4 receptor assessed as inhibition of binding of alkaline phosphatase-fused ephrin-A5 to immobilized EphA4 Fc by... |
ACS Med Chem Lett 7: 841-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00132
BindingDB Entry DOI: 10.7270/Q2TT4VF1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4 [1-949,A922V]
(Homo sapiens (Human)) | BDBM11985

(4-(2,5-dimethyl-1H-pyrrol-1-yl)-2-hydroxybenzoic a...)Show InChI InChI=1S/C13H13NO3/c1-8-3-4-9(2)14(8)10-5-6-11(13(16)17)12(15)7-10/h3-7,15H,1-2H3,(H,16,17) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Sanford-Burnham Medical Research Institute
| Assay Description
Protein A-coasted wells (from Pierce Biotechnology) were used to immobilize Eph receptor Fc fusion proteins (from R&D systems)incubated at 1 ug/ml in... |
Chem Biol Drug Des 78: 667-78 (2011)
Article DOI: 10.1111/j.1747-0285.2011.01199.x
BindingDB Entry DOI: 10.7270/Q2FN14QN |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50359971
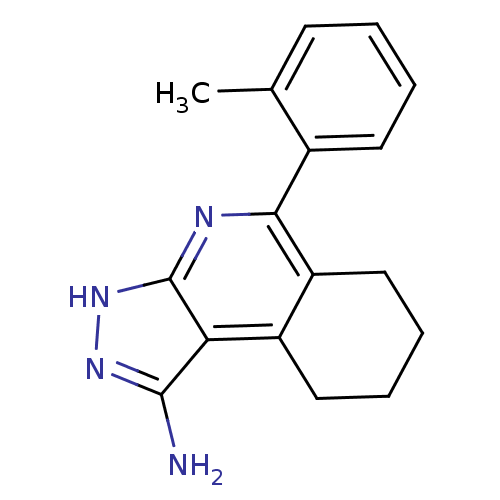
(CHEMBL1927263)Show InChI InChI=1S/C17H18N4/c1-10-6-2-3-7-11(10)15-13-9-5-4-8-12(13)14-16(18)20-21-17(14)19-15/h2-3,6-7H,4-5,8-9H2,1H3,(H3,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Inhibition of his-tagged EPHA4-mediated poly(Glu,Tyr) phosphorylation after 75 mins using E4:Y1 as substrate by fluorescence assay |
Eur J Med Chem 47: 493-500 (2012)
Article DOI: 10.1016/j.ejmech.2011.11.020
BindingDB Entry DOI: 10.7270/Q26110R2 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4 [1-949,A922V]
(Homo sapiens (Human)) | BDBM82187

(Salicylic acid derivative, 76D10)Show SMILES OC(=O)c1cc(ccc1O)-c1ccc(\C=C\C(=O)\C=C\c2ccc(o2)-c2ccc(O)c(c2)C(O)=O)o1 Show InChI InChI=1S/C27H18O9/c28-17(3-5-18-7-11-24(35-18)15-1-9-22(29)20(13-15)26(31)32)4-6-19-8-12-25(36-19)16-2-10-23(30)21(14-16)27(33)34/h1-14,29-30H,(H,31,32)(H,33,34)/b5-3+,6-4+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Sanford-Burnham Medical Research Institute
| Assay Description
Protein A-coasted wells (from Pierce Biotechnology) were used to immobilize Eph receptor Fc fusion proteins (from R&D systems)incubated at 1 ug/ml in... |
Chem Biol Drug Des 78: 667-78 (2011)
Article DOI: 10.1111/j.1747-0285.2011.01199.x
BindingDB Entry DOI: 10.7270/Q2FN14QN |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50428059
(CHEMBL2322989)Show SMILES C[C@H](CCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C |r| Show InChI InChI=1S/C35H50N2O4/c1-21(8-13-32(39)37-31(33(40)41)18-22-20-36-30-7-5-4-6-25(22)30)27-11-12-28-26-10-9-23-19-24(38)14-16-34(23,2)29(26)15-17-35(27,28)3/h4-7,20-21,23-24,26-29,31,36,38H,8-19H2,1-3H3,(H,37,39)(H,40,41)/t21-,23-,24-,26+,27-,28+,29+,31+,34+,35-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Parma
Curated by ChEMBL
| Assay Description
Displacement of ephrin-A1-Fc from EphA4 receptor Fc ectodomain (unknown origin) after 1 hr by ELISA |
J Med Chem 56: 2936-47 (2013)
Article DOI: 10.1021/jm301890k
BindingDB Entry DOI: 10.7270/Q2JW8G7H |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50359970

(CHEMBL1927262)Show InChI InChI=1S/C17H17FN4/c1-9-6-7-10(18)8-13(9)15-12-5-3-2-4-11(12)14-16(19)21-22-17(14)20-15/h6-8H,2-5H2,1H3,(H3,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Inhibition of his-tagged EPHA4-mediated poly(Glu,Tyr) phosphorylation after 75 mins using E4:Y1 as substrate by fluorescence assay |
Eur J Med Chem 47: 493-500 (2012)
Article DOI: 10.1016/j.ejmech.2011.11.020
BindingDB Entry DOI: 10.7270/Q26110R2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50157881
(CHEMBL3787614)Show SMILES Nc1ncnc2n(cc(-c3cccc(OC[C@@H]4CCCO4)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:23.26,16.15,25.29,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.55,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.98,9.12,;-1.51,10.57,;-.3,11.52,;.98,10.66,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C25H31N5O2/c26-24-23-22(18-4-1-5-20(12-18)32-15-21-6-2-9-31-21)14-30(25(23)28-16-27-24)19-10-17(11-19)13-29-7-3-8-29/h1,4-5,12,14,16-17,19,21H,2-3,6-11,13,15H2,(H2,26,27,28)/t17-,19+,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EphA4 (unknown origin) in presence of [gamma33P]ATP |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50359969

(CHEMBL1927261)Show InChI InChI=1S/C16H15ClN4/c17-12-8-4-3-7-11(12)14-10-6-2-1-5-9(10)13-15(18)20-21-16(13)19-14/h3-4,7-8H,1-2,5-6H2,(H3,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Inhibition of his-tagged EPHA4-mediated poly(Glu,Tyr) phosphorylation after 75 mins using E4:Y1 as substrate by fluorescence assay |
Eur J Med Chem 47: 493-500 (2012)
Article DOI: 10.1016/j.ejmech.2011.11.020
BindingDB Entry DOI: 10.7270/Q26110R2 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant EPHA4 |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50335374
((3S,9S)-9,16-dihydroxy-14-methoxy-3-methyl-3,4,9,1...)Show SMILES COc1cc(O)c2c(CCC[C@H](O)CC(=O)C=CC[C@H](C)OC2=O)c1 |r,w:17.17| Show InChI InChI=1S/C19H24O6/c1-12-5-3-7-14(20)10-15(21)8-4-6-13-9-16(24-2)11-17(22)18(13)19(23)25-12/h3,7,9,11-12,15,21-22H,4-6,8,10H2,1-2H3/t12-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of EPHA4 by TR-FRET based LanthaScreen assay |
ACS Med Chem Lett 2: 22-27 (2011)
Article DOI: 10.1021/ml1001807
BindingDB Entry DOI: 10.7270/Q2542NWT |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50363167

(CHEMBL1945559)Show SMILES Cn1c2c(C(C#N)C3(CCNCC3)NC2=O)c2ccc(Cl)c(Cl)c12 Show InChI InChI=1S/C17H16Cl2N4O/c1-23-14-9(2-3-11(18)13(14)19)12-10(8-20)17(4-6-21-7-5-17)22-16(24)15(12)23/h2-3,10,21H,4-7H2,1H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University of Munich
Curated by ChEMBL
| Assay Description
Inhibition of EphA4 using ATP as substrate |
J Med Chem 55: 403-13 (2012)
Article DOI: 10.1021/jm201286z
BindingDB Entry DOI: 10.7270/Q20G3KK7 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50462709
(CHEMBL4245507)Show SMILES O=C(Cc1cccc(OCCCN2CCNCC2)c1)Nc1nc(cs1)-c1ccncc1 Show InChI InChI=1S/C23H27N5O2S/c29-22(27-23-26-21(17-31-23)19-5-7-24-8-6-19)16-18-3-1-4-20(15-18)30-14-2-11-28-12-9-25-10-13-28/h1,3-8,15,17,25H,2,9-14,16H2,(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of EphA4 (unknown origin) |
Bioorg Med Chem Lett 28: 2616-2621 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.043
BindingDB Entry DOI: 10.7270/Q2XP77MH |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50535180
(CHEMBL4468291)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)CCN |r| Show InChI InChI=1S/C67H91N17O18S2/c1-35(2)56-66(102)80-47(30-37-13-17-40(86)18-14-37)61(97)76-43(9-5-26-73-67(70)71)58(94)72-27-24-52(87)75-44(19-21-54(89)90)59(95)77-45(20-22-55(91)92)60(96)78-48(31-38-32-74-42-8-4-3-7-41(38)42)63(99)81-49(57(69)93)33-103-104-34-50(64(100)83-56)82-62(98)46(29-36-11-15-39(85)16-12-36)79-65(101)51-10-6-28-84(51)53(88)23-25-68/h3-4,7-8,11-18,32,35,43-51,56,74,85-86H,5-6,9-10,19-31,33-34,68H2,1-2H3,(H2,69,93)(H,72,94)(H,75,87)(H,76,97)(H,77,95)(H,78,96)(H,79,101)(H,80,102)(H,81,99)(H,82,98)(H,83,100)(H,89,90)(H,91,92)(H4,70,71,73)/t43-,44-,45-,46-,47-,48-,49-,50-,51-,56-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against human EphA4 receptor assessed as inhibition of binding of alkaline phosphatase-fused ephrin-A5 to immobilized EphA4 Fc by... |
ACS Med Chem Lett 7: 841-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00132
BindingDB Entry DOI: 10.7270/Q2TT4VF1 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50519662
(CHEMBL4438748)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1cccc(NC(=O)COc2ccc3c(c2)occc3=O)c1 Show InChI InChI=1S/C26H23N3O5/c1-29(2)20-8-6-17(7-9-20)26(32)28-19-5-3-4-18(14-19)27-25(31)16-34-21-10-11-22-23(30)12-13-33-24(22)15-21/h3-15H,16H2,1-2H3,(H,27,31)(H,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human EphA4 (601 to 892 residues) using poly(Glu, Tyr) 4:1 as substrate measured after 40 mins in presence of [gamma33ATP] ... |
J Med Chem 62: 10691-10710 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01143
BindingDB Entry DOI: 10.7270/Q2MC93FG |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM106870
(US8592455, 70)Show SMILES C[C@@H]1C[C@H](N)C[C@@H](C1)c1ccncc1NC(=O)c1ccc(F)c(n1)-c1c(F)cccc1F |r,wU:6.8,3.3,1.0,(-.67,2.69,;-2,1.93,;-3.33,2.69,;-4.67,1.93,;-6,2.69,;-4.67,.38,;-3.33,-.38,;-2,.38,;-3.33,-1.93,;-4.67,-2.69,;-4.67,-4.23,;-3.33,-5,;-2,-4.23,;-2,-2.69,;-.67,-1.93,;.67,-2.69,;.67,-4.23,;2,-1.93,;3.33,-2.69,;4.67,-1.93,;4.67,-.38,;6,.38,;3.33,.38,;2,-.38,;3.33,1.93,;4.67,2.69,;6,1.93,;4.67,4.23,;3.33,5,;2,4.23,;2,2.69,;.67,1.93,)| Show InChI InChI=1S/C24H23F3N4O/c1-13-9-14(11-15(28)10-13)16-7-8-29-12-21(16)31-24(32)20-6-5-19(27)23(30-20)22-17(25)3-2-4-18(22)26/h2-8,12-15H,9-11,28H2,1H3,(H,31,32)/t13-,14+,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EPHA4 (unknown origin) |
J Med Chem 58: 8373-86 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01275
BindingDB Entry DOI: 10.7270/Q2H41VGN |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50413752
(CHEMBL2012519 | L-783277)Show SMILES COc1cc(O)c2c(CCCC(=O)C(=O)C(O)CCC[C@H](C)OC2=O)c1 |r| Show InChI InChI=1S/C19H24O7/c1-11-5-3-7-14(20)18(23)15(21)8-4-6-12-9-13(25-2)10-16(22)17(12)19(24)26-11/h9-11,14,20,22H,3-8H2,1-2H3/t11-,14?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of EPHA4 by TR-FRET based LanthaScreen assay |
ACS Med Chem Lett 2: 22-27 (2011)
Article DOI: 10.1021/ml1001807
BindingDB Entry DOI: 10.7270/Q2542NWT |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM107057

(US8592432, 34)Show SMILES CC(C)Oc1cc(C2CCN(C)CC2)c(C)cc1Nc1nc(Nc2ccccc2S(=O)(=O)C(C)C)c2c(C)[nH]nc2n1 Show InChI InChI=1S/C31H41N7O3S/c1-18(2)41-26-17-23(22-12-14-38(7)15-13-22)20(5)16-25(26)33-31-34-29(28-21(6)36-37-30(28)35-31)32-24-10-8-9-11-27(24)42(39,40)19(3)4/h8-11,16-19,22H,12-15H2,1-7H3,(H3,32,33,34,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EphA4 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2015.11.049
BindingDB Entry DOI: 10.7270/Q2XS603J |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4 [1-949,A922V]
(Homo sapiens (Human)) | BDBM82190
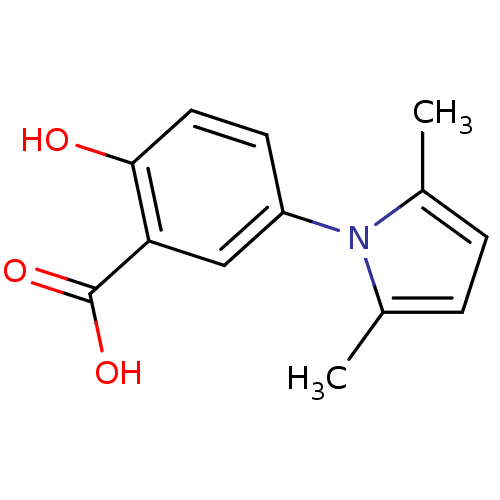
(Salicylic acid derivative, compound 2)Show InChI InChI=1S/C13H13NO3/c1-8-3-4-9(2)14(8)10-5-6-12(15)11(7-10)13(16)17/h3-7,15H,1-2H3,(H,16,17) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Sanford-Burnham Medical Research Institute
| Assay Description
Protein A-coasted wells (from Pierce Biotechnology) were used to immobilize Eph receptor Fc fusion proteins (from R&D systems)incubated at 1 ug/ml in... |
Chem Biol Drug Des 78: 667-78 (2011)
Article DOI: 10.1111/j.1747-0285.2011.01199.x
BindingDB Entry DOI: 10.7270/Q2FN14QN |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50157879
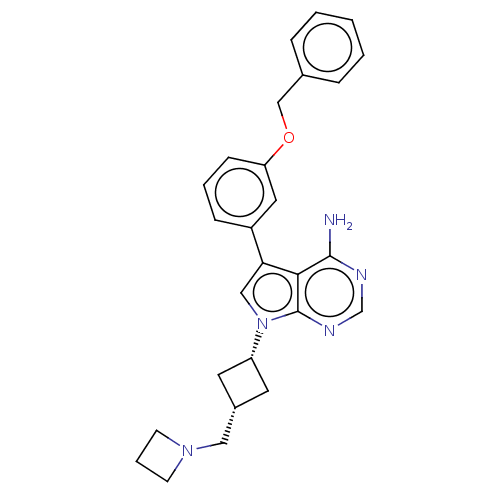
(CHEMBL1614712)Show SMILES [H][C@]1(CN2CCC2)C[C@]([H])(C1)n1cc(-c2cccc(OCc3ccccc3)c2)c2c(N)ncnc12 |wD:8.9,1.0,(6.47,-15.11,;5.71,-13.78,;4.93,-15.11,;5.69,-16.44,;5.68,-17.98,;7.22,-17.99,;7.23,-16.45,;5.31,-12.3,;6.81,-11.9,;6.02,-10.55,;7.2,-13.39,;7.59,-10.56,;6.61,-9.37,;7.45,-8.07,;7.04,-6.58,;5.56,-6.19,;5.15,-4.71,;6.24,-3.6,;7.73,-3.99,;8.81,-2.89,;10.15,-3.66,;11.48,-2.89,;12.81,-3.66,;14.12,-2.9,;14.13,-1.36,;12.8,-.59,;11.48,-1.36,;8.13,-5.47,;8.94,-8.47,;10.35,-7.7,;10.34,-6.15,;11.69,-8.46,;11.69,-10.01,;10.35,-10.79,;9.02,-10.01,)| Show InChI InChI=1S/C27H29N5O/c28-26-25-24(21-8-4-9-23(14-21)33-17-19-6-2-1-3-7-19)16-32(27(25)30-18-29-26)22-12-20(13-22)15-31-10-5-11-31/h1-4,6-9,14,16,18,20,22H,5,10-13,15,17H2,(H2,28,29,30)/t20-,22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EphA4 (unknown origin) in presence of [gamma33P]ATP |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50158429

(CHEMBL3785500)Show SMILES Nc1ncnc2n(cc(-c3cccc(OCC45CCC(CC4)O5)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:25.29,27.32,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.92,;4.34,4.35,;3.39,5.56,;1.86,5.34,;.91,6.56,;1.49,7.99,;.54,9.2,;-.78,8.6,;-1.87,9.66,;-1.5,11.15,;-.02,11.55,;1.07,10.5,;-.08,10.34,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.26,;4.58,-6.69,;5.27,-8.03,;6.62,-7.29,;5.88,-5.94,;1.42,-3.96,)| Show InChI InChI=1S/C27H33N5O2/c28-25-24-23(19-3-1-4-22(13-19)33-16-27-7-5-21(34-27)6-8-27)15-32(26(24)30-17-29-25)20-11-18(12-20)14-31-9-2-10-31/h1,3-4,13,15,17-18,20-21H,2,5-12,14,16H2,(H2,28,29,30)/t18-,20+,21?,27? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EphA4 (unknown origin) in presence of [gamma33P]ATP |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50157628
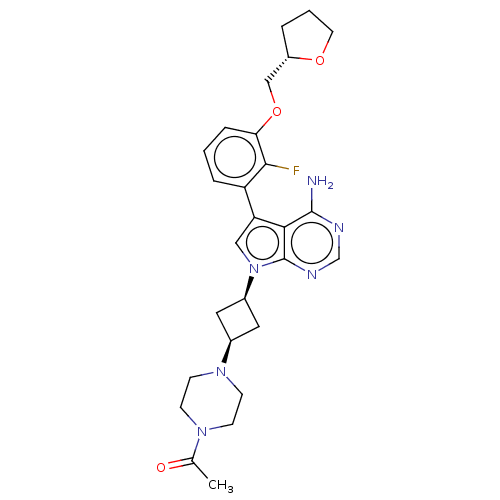
(CHEMBL3786167)Show SMILES CC(=O)N1CCN(CC1)[C@H]1C[C@H](C1)n1cc(-c2cccc(OC[C@@H]3CCCO3)c2F)c2c(N)ncnc12 |r,wD:11.14,23.24,9.9,(4.28,-11.46,;5,-10.46,;6.22,-10.59,;4.36,-9.06,;2.83,-8.9,;2.2,-7.5,;3.1,-6.26,;4.64,-6.41,;5.27,-7.81,;2.74,-4.76,;3.54,-3.45,;2.24,-2.7,;1.42,-3.96,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.55,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.98,9.12,;-1.51,10.57,;-.3,11.52,;.98,10.66,;1.29,3.91,;.07,3.74,;.3,.77,;-1.03,1.55,;-1.03,2.79,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C27H33FN6O3/c1-17(35)32-7-9-33(10-8-32)18-12-19(13-18)34-14-22(24-26(29)30-16-31-27(24)34)21-5-2-6-23(25(21)28)37-15-20-4-3-11-36-20/h2,5-6,14,16,18-20H,3-4,7-13,15H2,1H3,(H2,29,30,31)/t18-,19+,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EphA4 (unknown origin) in presence of [gamma33P]ATP |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 4
(Homo sapiens (Human)) | BDBM50156383

(CHEMBL3785951)Show SMILES CC(=O)N1CCN(CC1)[C@H]1C[C@H](C1)n1cc(-c2cc(OC[C@@H]3CCCO3)ccc2F)c2c(N)ncnc12 |r,wU:21.22,wD:11.14,9.9,(4.28,-11.46,;5,-10.46,;6.22,-10.59,;4.36,-9.06,;2.83,-8.9,;2.2,-7.5,;3.1,-6.26,;4.64,-6.41,;5.27,-7.81,;2.74,-4.76,;3.54,-3.45,;2.24,-2.7,;1.42,-3.96,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;5.86,4.56,;6.43,5.99,;7.96,6.21,;9.01,5.1,;10.39,5.78,;10.17,7.31,;8.66,7.57,;3.38,5.55,;1.86,5.34,;1.29,3.91,;.07,3.74,;.3,.77,;-1.03,1.55,;-1.03,2.79,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C27H33FN6O3/c1-17(35)32-6-8-33(9-7-32)18-11-19(12-18)34-14-23(25-26(29)30-16-31-27(25)34)22-13-20(4-5-24(22)28)37-15-21-3-2-10-36-21/h4-5,13-14,16,18-19,21H,2-3,6-12,15H2,1H3,(H2,29,30,31)/t18-,19+,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EphA4 (unknown origin) in presence of [gamma33P]ATP |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data