

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 9 [277-582] (Homo sapiens (Human)) | BDBM231167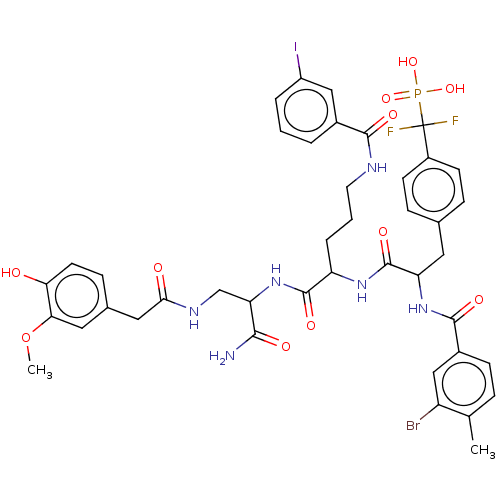 (US9340574, 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 34 | n/a | 75 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in 3,3-dimethylglutarate buffer (50 mM 3,3-dimethylglutarate, pH 7.0, 1 ... | US Patent US9340574 (2016) BindingDB Entry DOI: 10.7270/Q2NV9H4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 [277-582] (Homo sapiens (Human)) | BDBM231166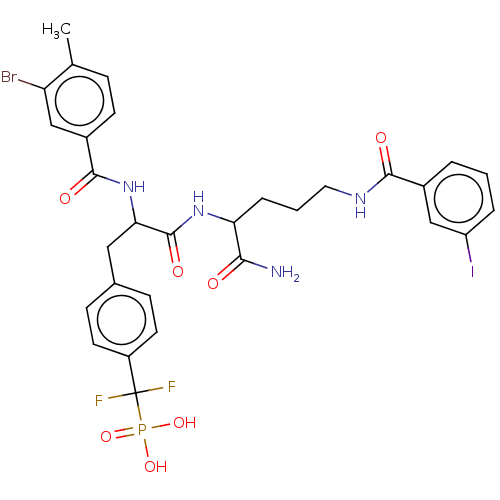 (US9340574, 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 270 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in 3,3-dimethylglutarate buffer (50 mM 3,3-dimethylglutarate, pH 7.0, 1 ... | US Patent US9340574 (2016) BindingDB Entry DOI: 10.7270/Q2NV9H4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 [277-582] (Homo sapiens (Human)) | BDBM231165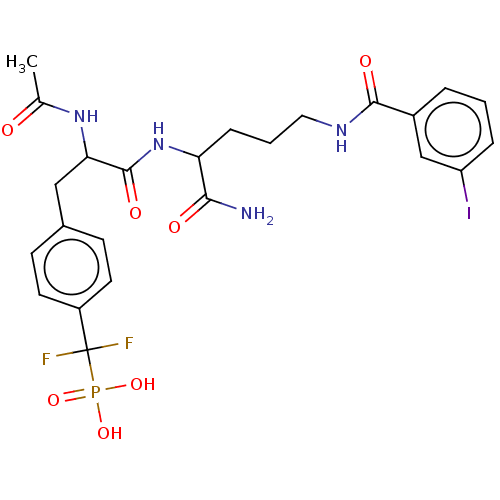 (US9340574, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 900 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in 3,3-dimethylglutarate buffer (50 mM 3,3-dimethylglutarate, pH 7.0, 1 ... | US Patent US9340574 (2016) BindingDB Entry DOI: 10.7270/Q2NV9H4J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||