

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107727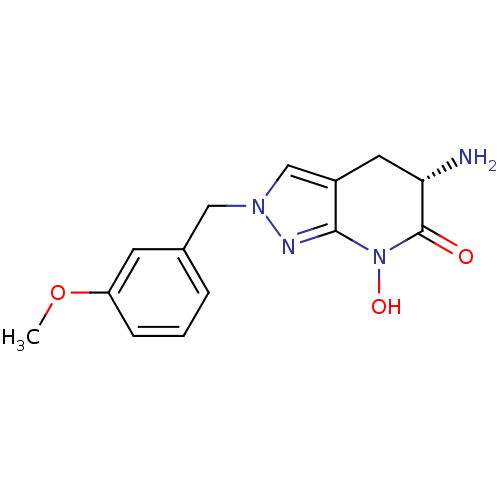 (US8933095, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.93 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107721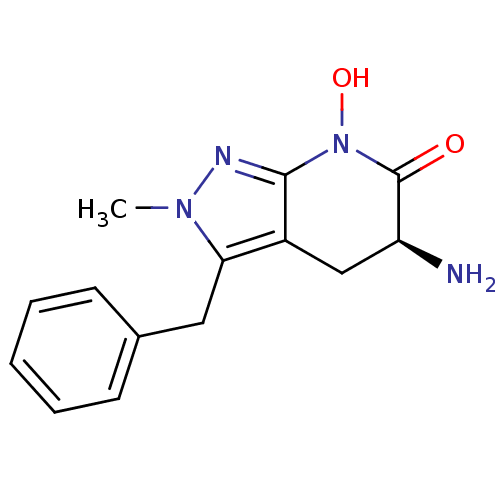 (US8933095, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107747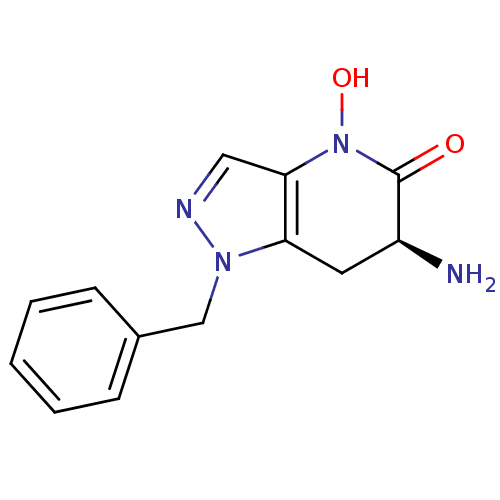 (CHEMBL2347115 | US8933095, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 22.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107739 (US8933095, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 24.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107730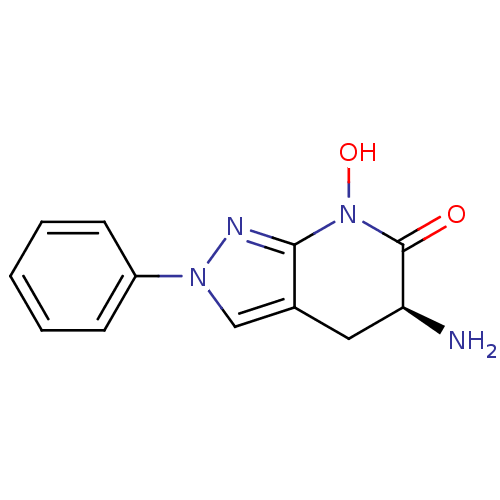 (CHEMBL2347108 | US8933095, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 24.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107731 (US8933095, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 28.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107745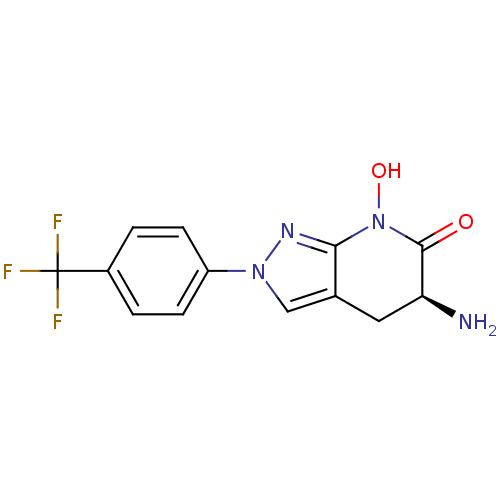 (US8933095, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107724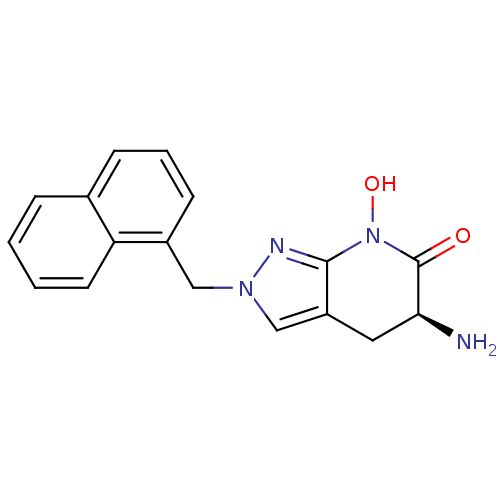 (US8933095, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107743 (US8933095, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107744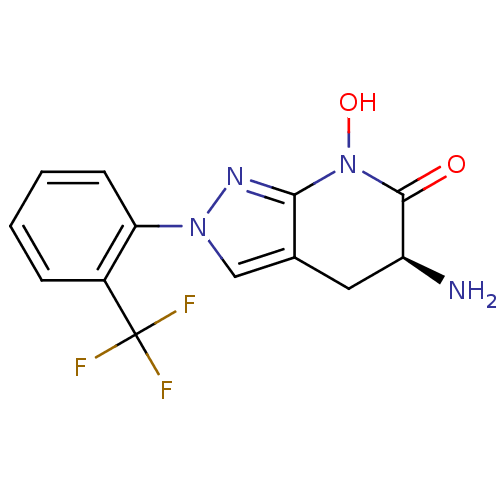 (US8933095, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 38.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107742 (US8933095, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107741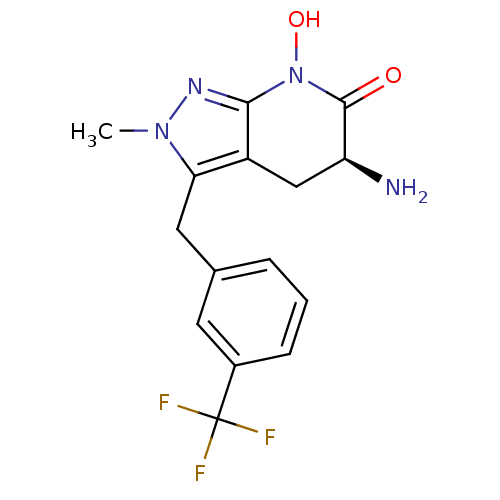 (US8933095, 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 42.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107723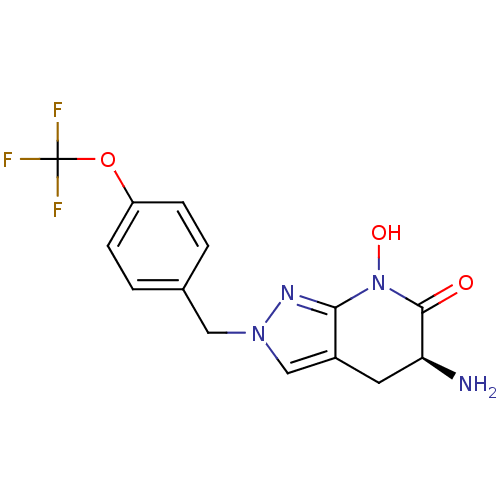 (US8933095, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 43.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107722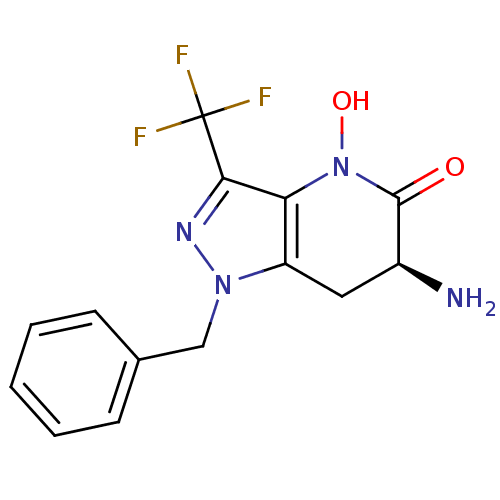 (CHEMBL2347114 | US8933095, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 43.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107726 (US8933095, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107746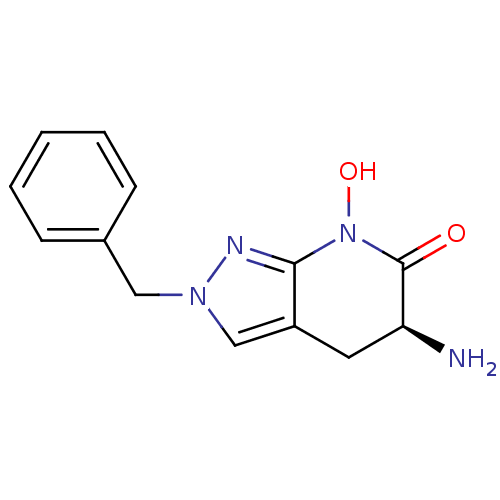 (CHEMBL2347107 | US8933095, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 59.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107740 (US8933095, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107720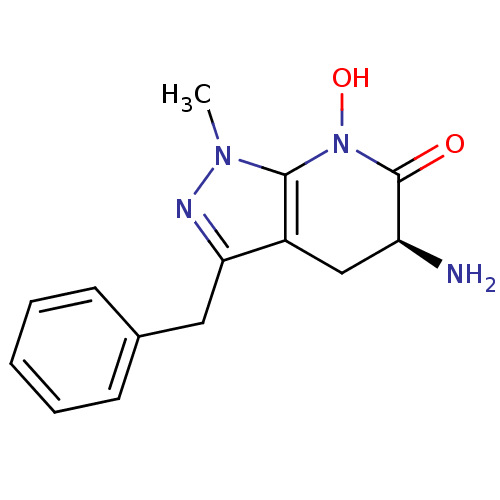 (CHEMBL2347112 | US8598200, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 63.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107738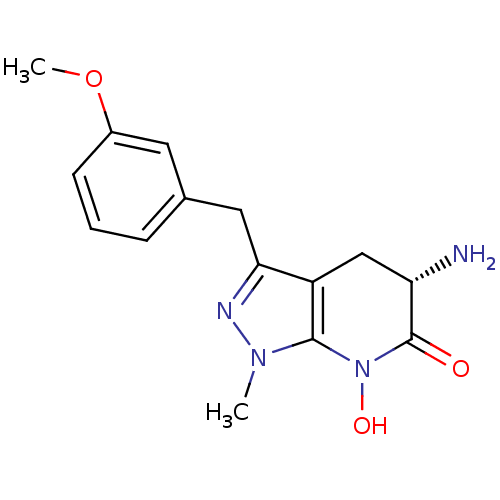 (CHEMBL2347113 | US8933095, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 65.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107748 (US8933095, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 74.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107735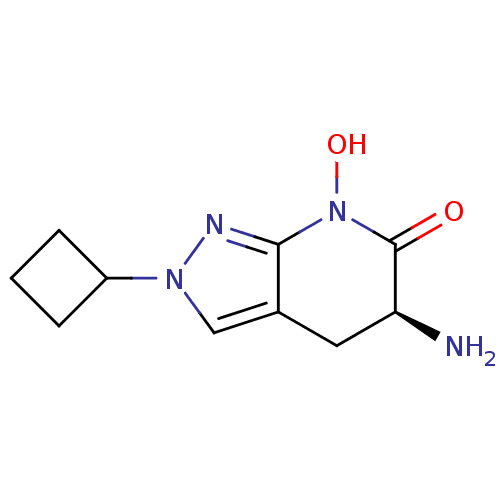 (US8933095, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 81.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107728 (US8933095, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 81.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107732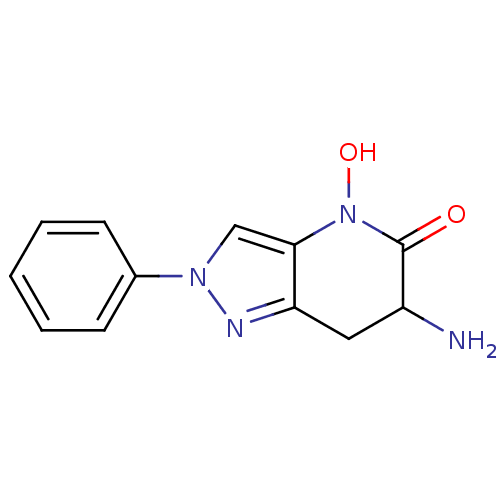 (US8598200, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107725 (US8933095, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107736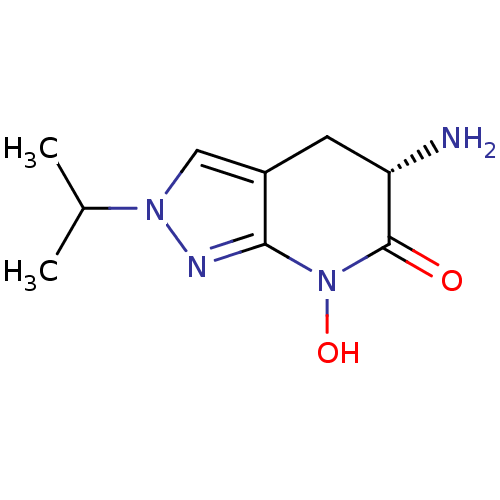 (US8933095, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107729 (US8933095, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 182 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107734 (CHEMBL2347111 | US8933095, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 329 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107737 (US8933095, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107733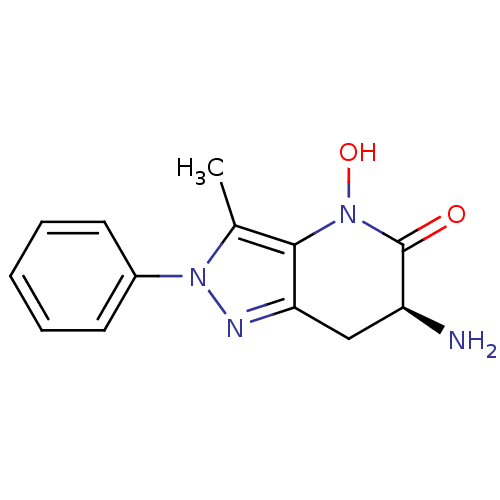 (US8933095, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||