

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM18313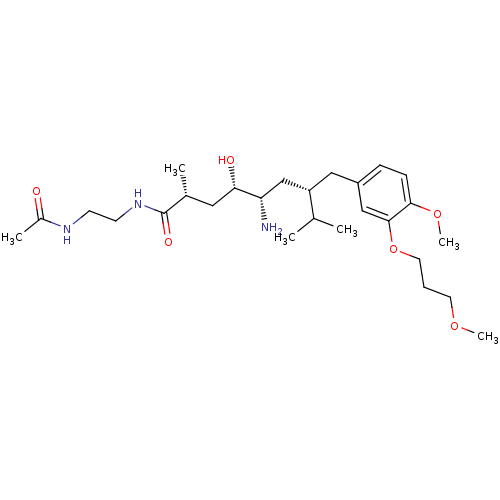 ((2R,4S,5S,7S)-5-amino-N-(2-acetamidoethyl)-4-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18288 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18278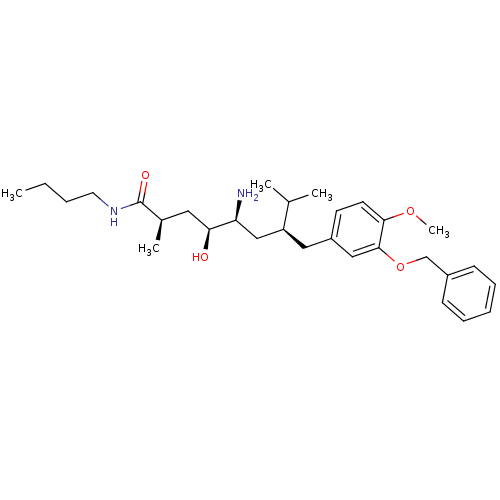 ((2R,4S,5S,7S)-5-amino-7-{[3-(benzyloxy)-4-methoxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18289 ((2R,4S,5S,7S)-5-amino-4-hydroxy-N-(4-hydroxybutyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18304 (8-phenyl-octanecarboxamide peptidomimetic, 61 | me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17949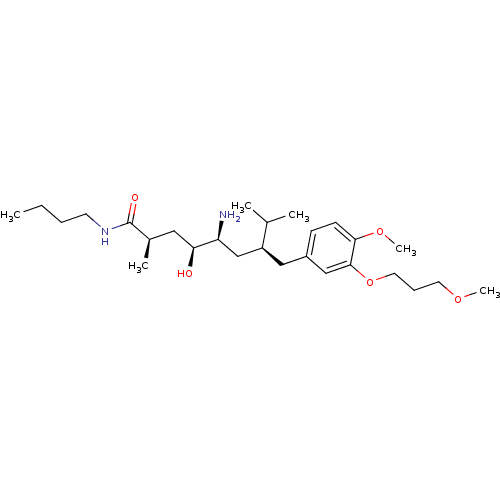 ((2R,4S,5S,7S)-5-amino-N-butyl-4-hydroxy-7-{[4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18306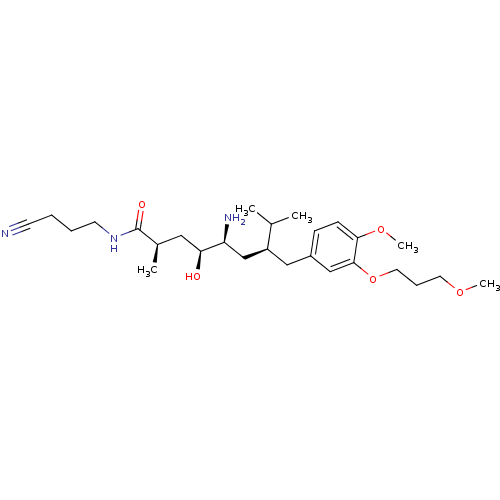 ((2R,4S,5S,7S)-5-amino-N-(3-cyanopropyl)-4-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18308 ((2R,4S,5S,7S)-5-amino-N-(3-carbamoylpropyl)-4-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18295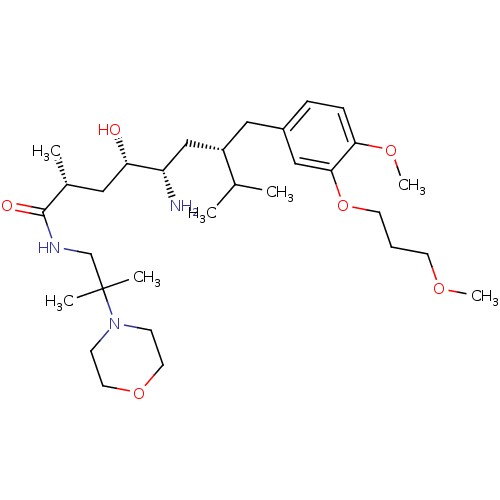 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18294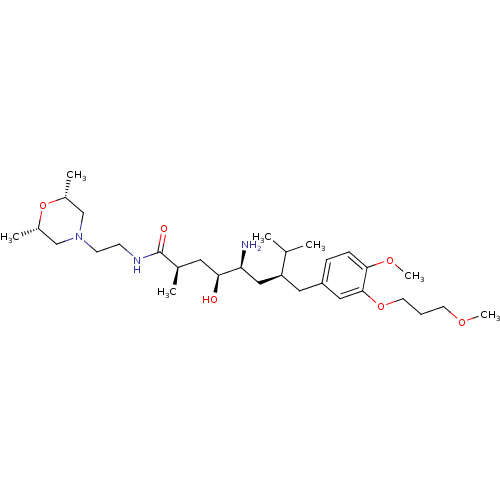 ((2R,4S,5S,7S)-5-amino-N-{2-[(2R,6S)-2,6-dimethylmo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18293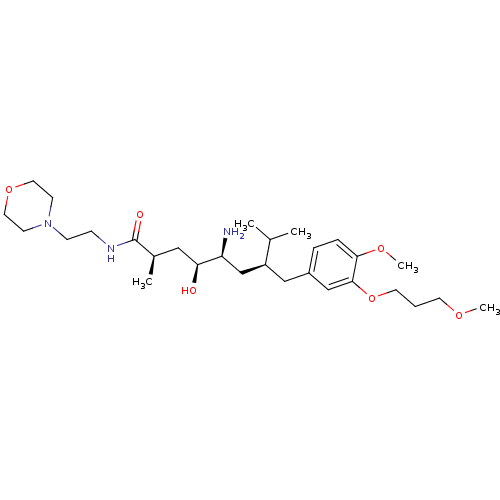 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18316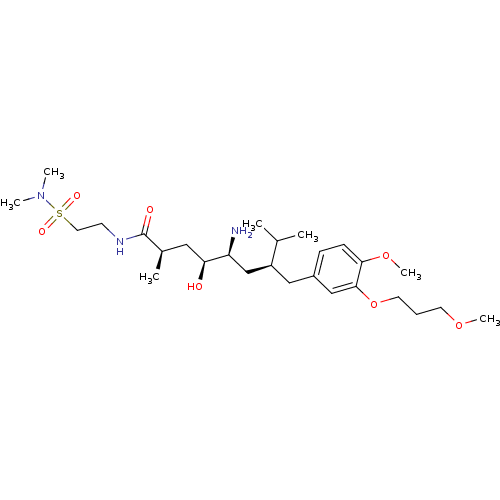 ((2R,4S,5S,7S)-5-amino-N-[2-(dimethylsulfamoyl)ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18284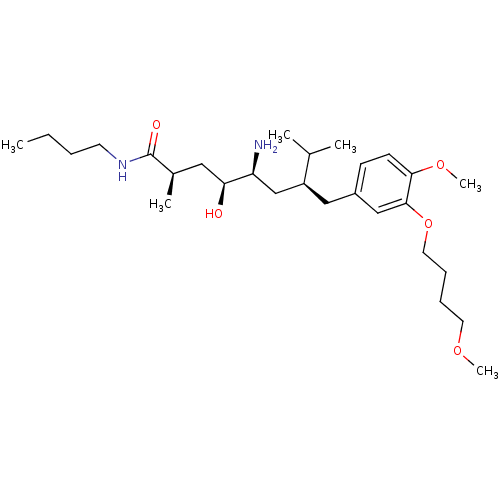 ((2R,4S,5S,7S)-5-amino-N-butyl-4-hydroxy-7-{[4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18297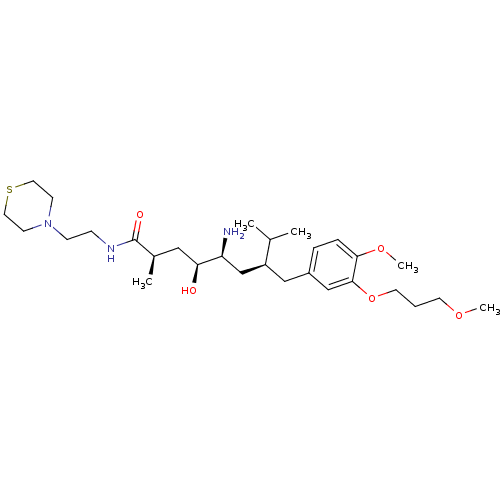 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18298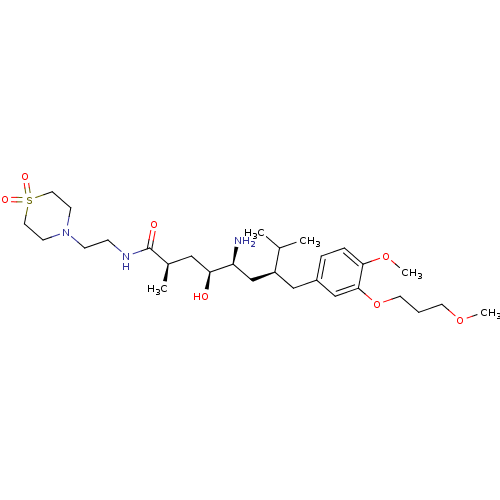 ((2R,4S,5S,7S)-5-amino-N-[2-(1,1-dioxo-1,4-thiomorp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18310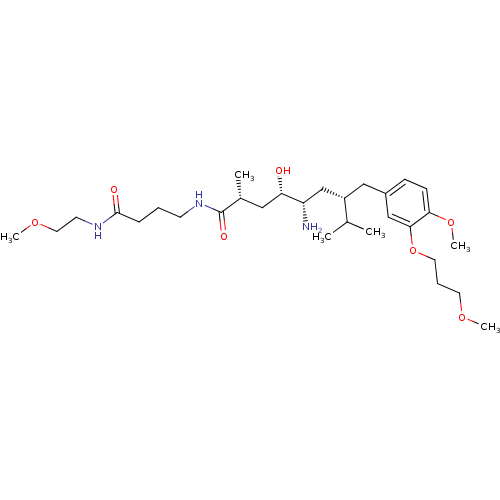 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18311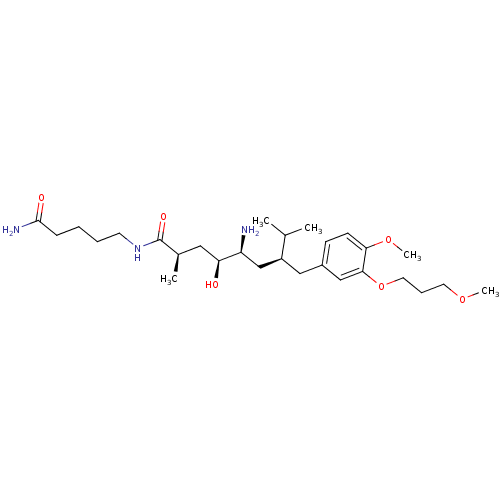 ((2R,4S,5S,7S)-5-amino-N-(4-carbamoylbutyl)-4-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18287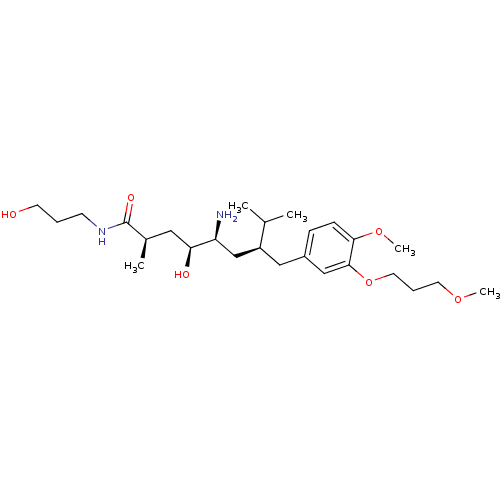 ((2R,4S,5S,7S)-5-amino-4-hydroxy-N-(3-hydroxypropyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18320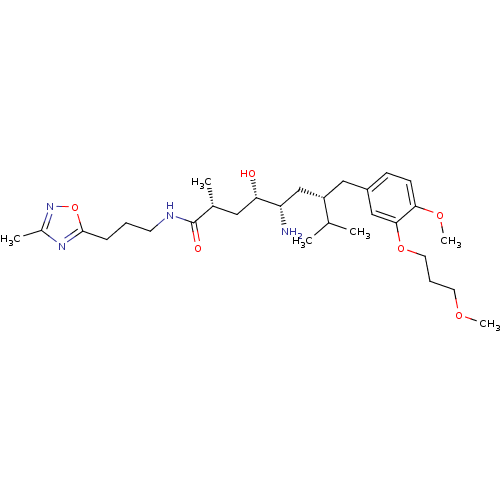 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18312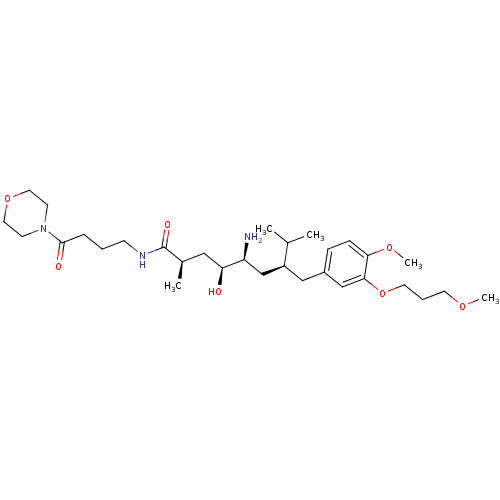 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18282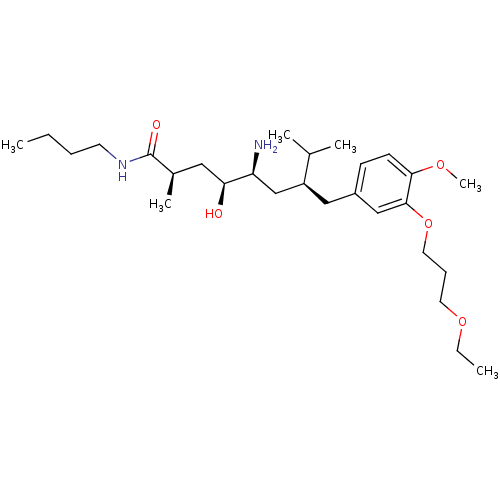 ((2R,4S,5S,7S)-5-amino-N-butyl-7-{[3-(3-ethoxypropo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18319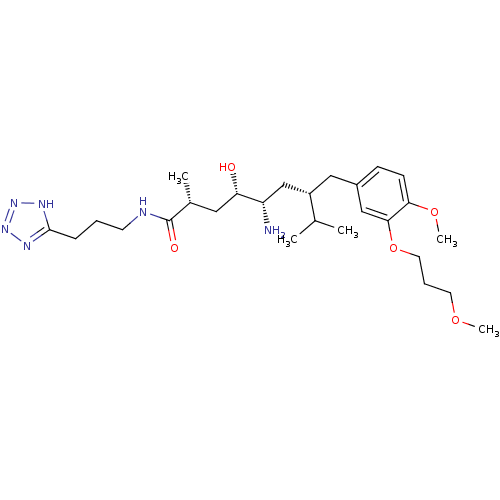 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18309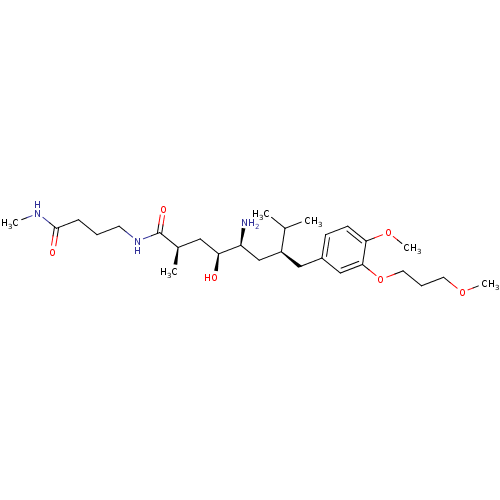 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18302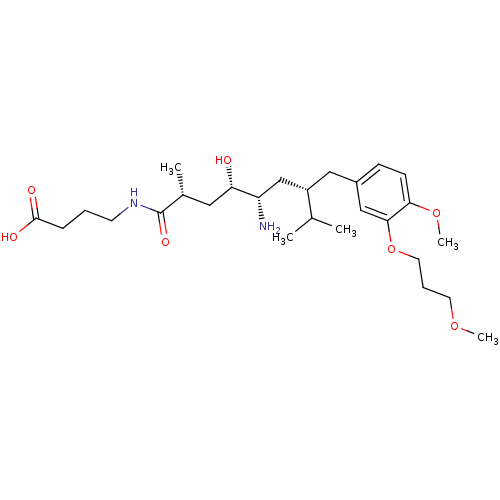 (4-[(2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18318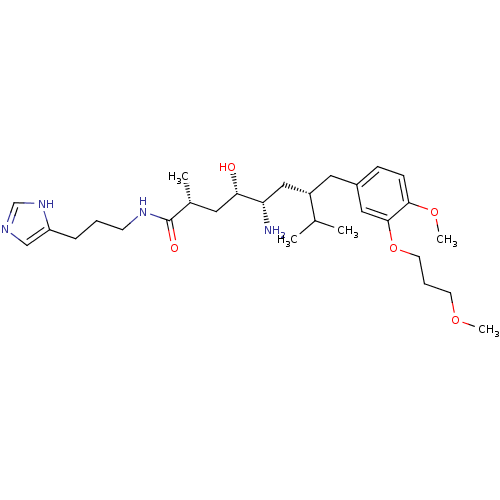 ((2R,4S,5S,7S)-5-amino-4-hydroxy-N-[3-(1H-imidazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18286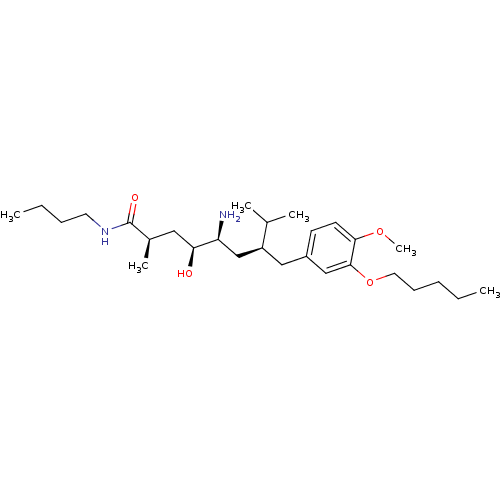 ((2R,4S,5S,7S)-5-amino-N-butyl-4-hydroxy-7-{[4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18270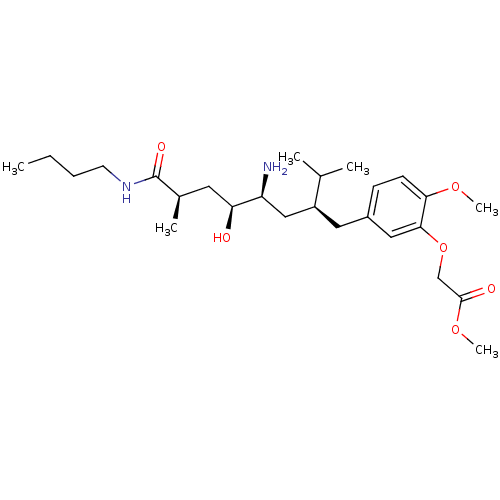 (8-phenyl-octanecarboxamide peptidomimetic, 28 | me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18314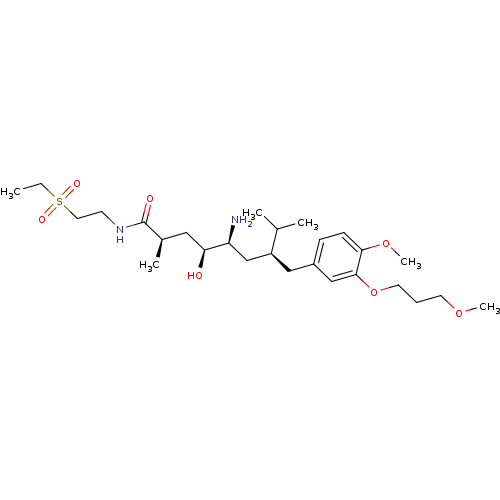 ((2R,4S,5S,7S)-5-amino-N-[2-(ethanesulfonyl)ethyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18280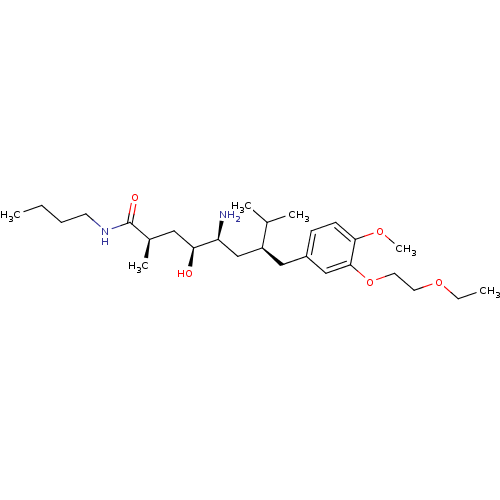 ((2R,4S,5S,7S)-5-amino-N-butyl-7-{[3-(2-ethoxyethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18303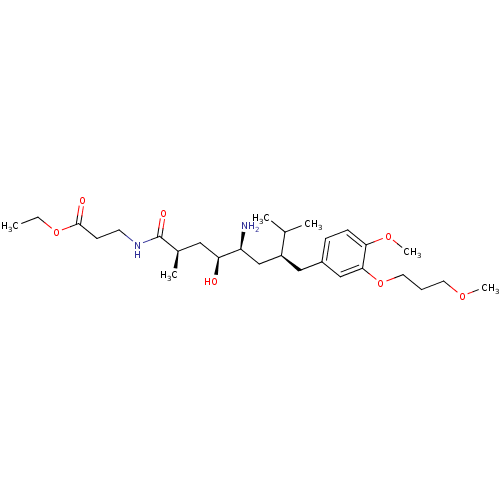 (8-phenyl-octanecarboxamide peptidomimetic, 60 | et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18277 ((2R,4S,5S,7S)-5-amino-N-butyl-4-hydroxy-7-{[4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18300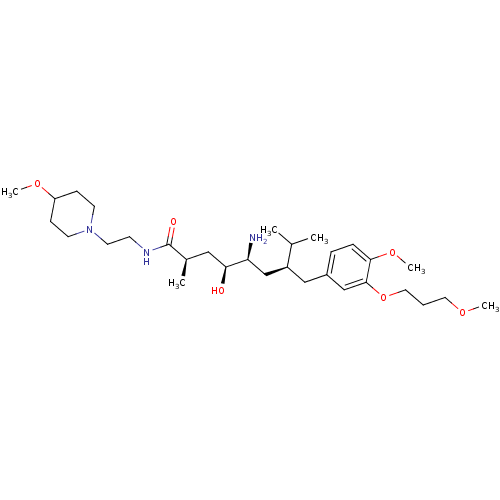 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18283 ((2R,4S,5S,7S)-5-amino-N-butyl-4-hydroxy-7-{[3-(3-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18269 (8-phenyl-octanecarboxamide peptidomimetic, 27 | me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18305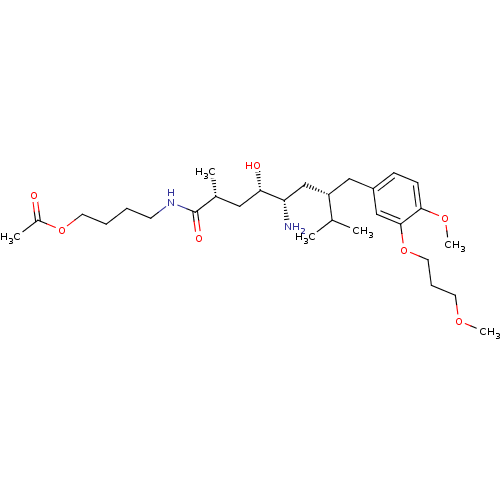 (4-[(2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18315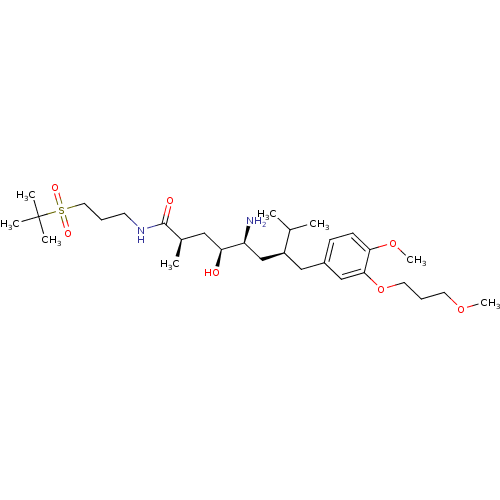 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18307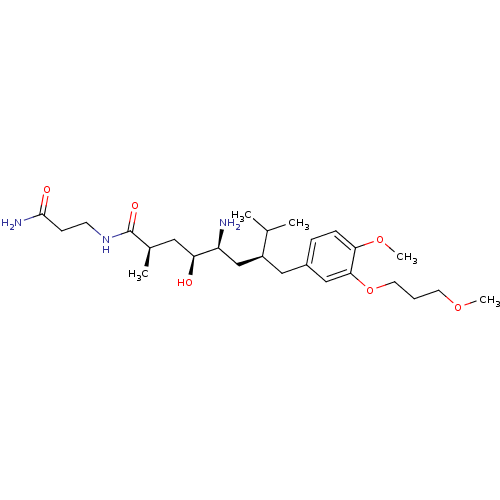 ((2R,4S,5S,7S)-5-amino-N-(2-carbamoylethyl)-4-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18317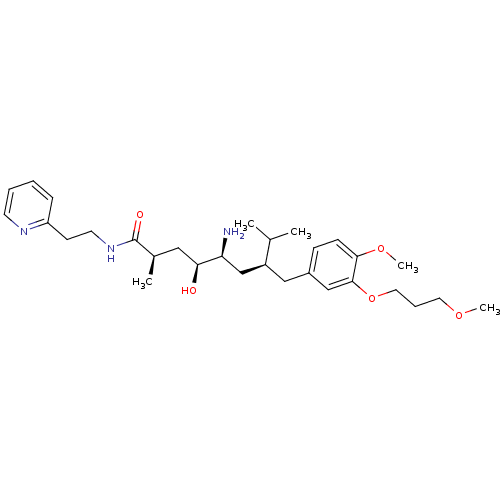 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18299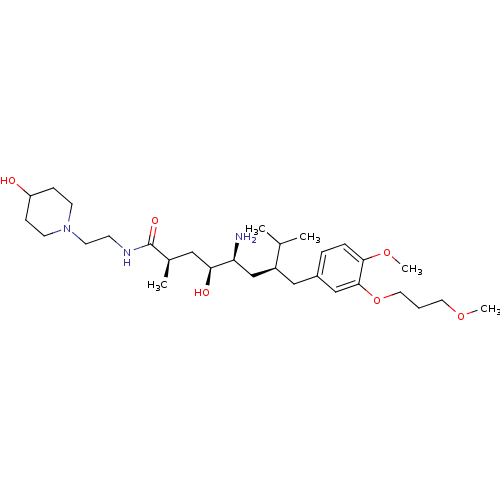 ((2R,4S,5S,7S)-5-amino-4-hydroxy-N-[2-(4-hydroxypip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18279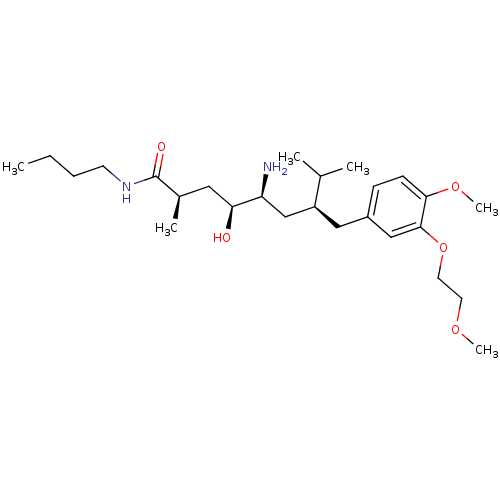 ((2R,4S,5S,7S)-5-amino-N-butyl-4-hydroxy-7-{[4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18296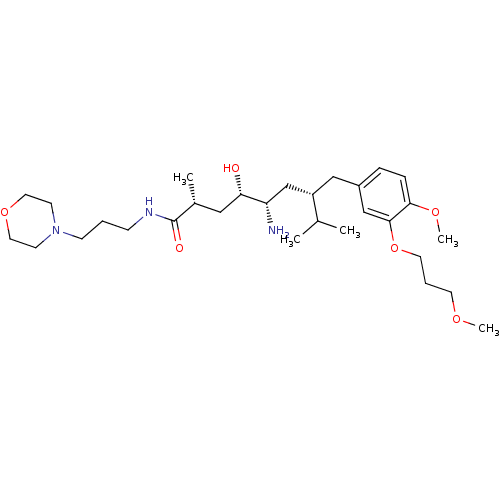 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18301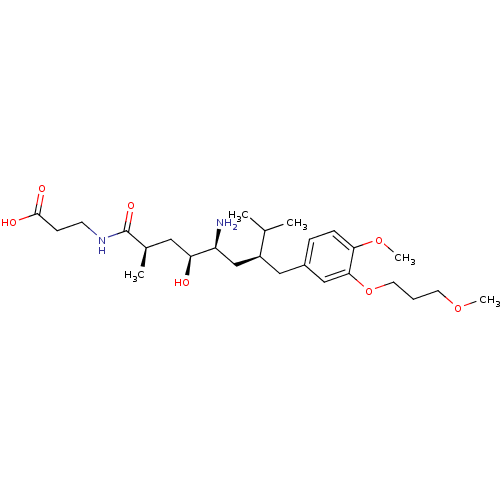 (3-[(2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18274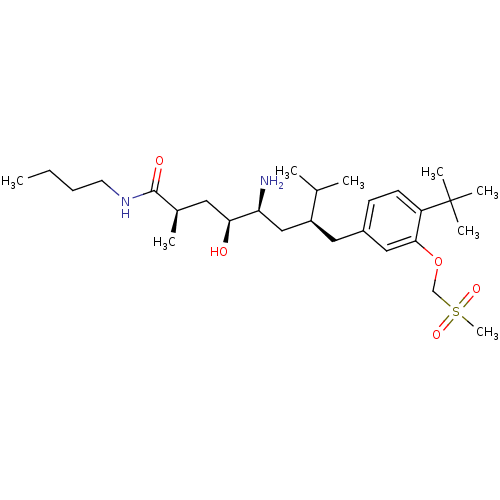 ((2R,4S,5S,7S)-5-amino-N-butyl-7-{[4-tert-butyl-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18291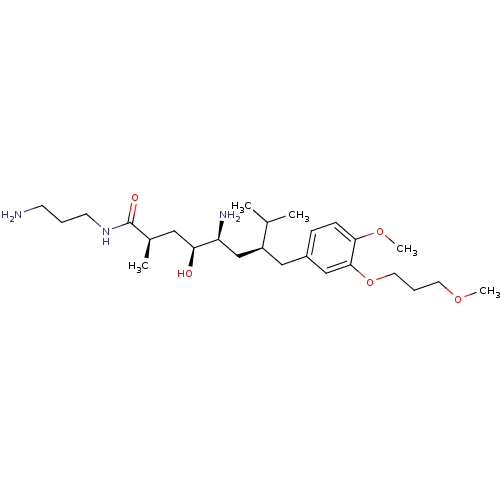 ((2R,4S,5S,7S)-5-amino-N-(3-aminopropyl)-4-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18290 ((2R,4S,5S,7S)-5-amino-N-[2-(dimethylamino)ethyl]-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18285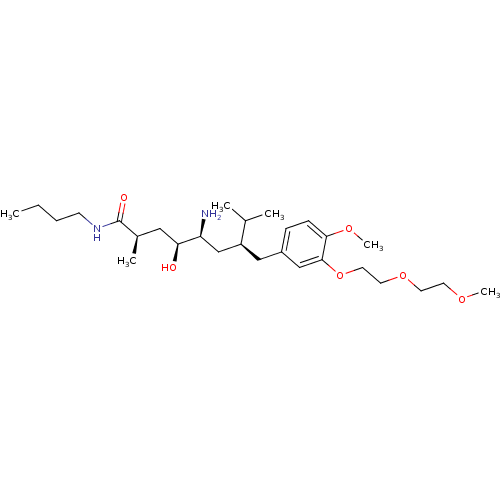 ((2R,4S,5S,7S)-5-amino-N-butyl-4-hydroxy-7-({4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18271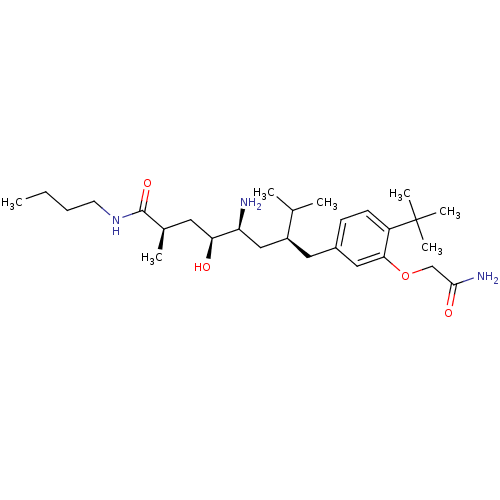 ((2R,4S,5S,7S)-5-amino-N-butyl-7-{[4-tert-butyl-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18292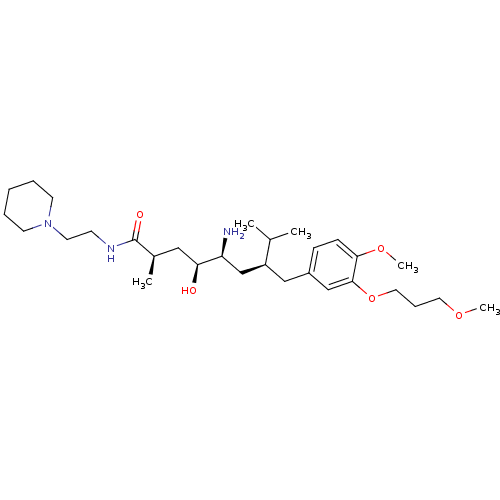 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18276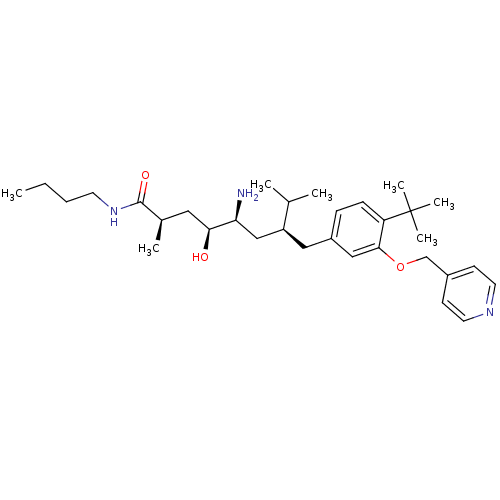 ((2R,4S,5S,7S)-5-amino-N-butyl-7-{[4-tert-butyl-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18273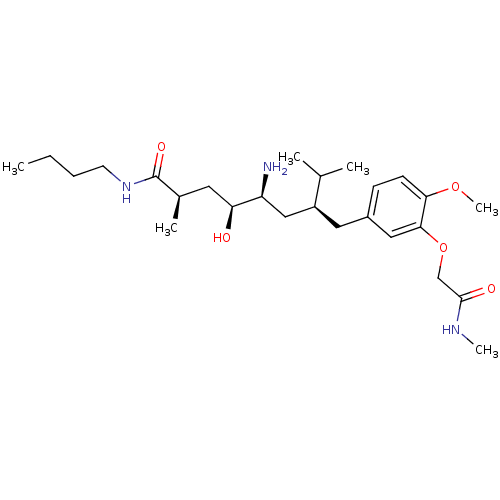 ((2R,4S,5S,7S)-5-amino-N-butyl-4-hydroxy-7-({4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 53 total ) | Next | Last >> |