

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM18342 ((2S,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18337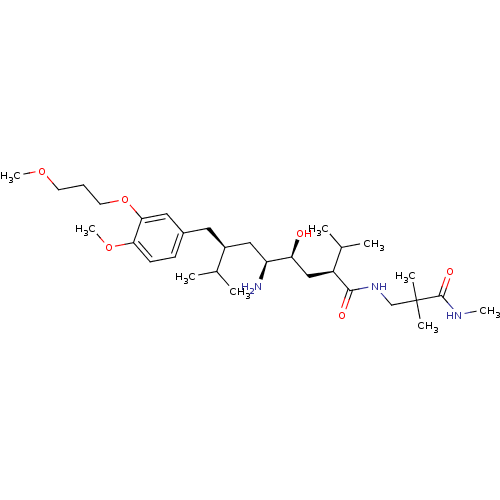 ((2S,4S,5S,7S)-5-amino-N-[2,2-dimethyl-2-(methylcar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18344 ((2S,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18340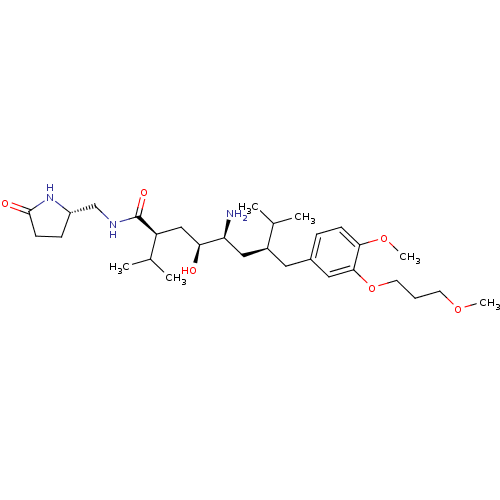 ((2S,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17950 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18323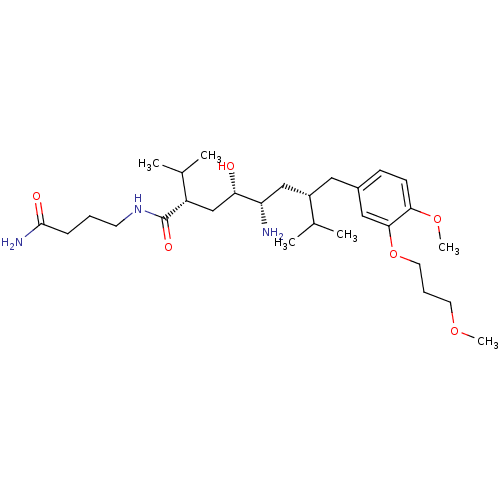 ((2S,4S,5S,7S)-5-amino-N-(3-carbamoylpropyl)-4-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18338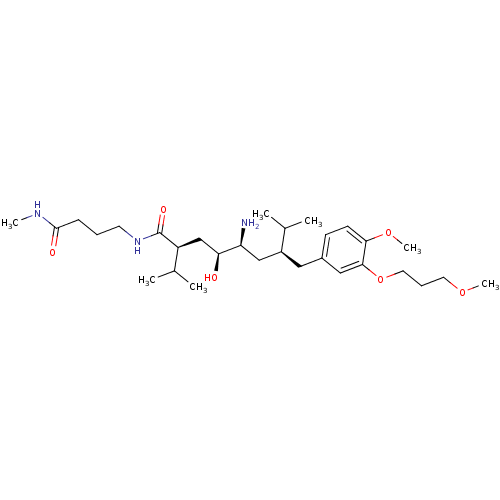 ((2S,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18326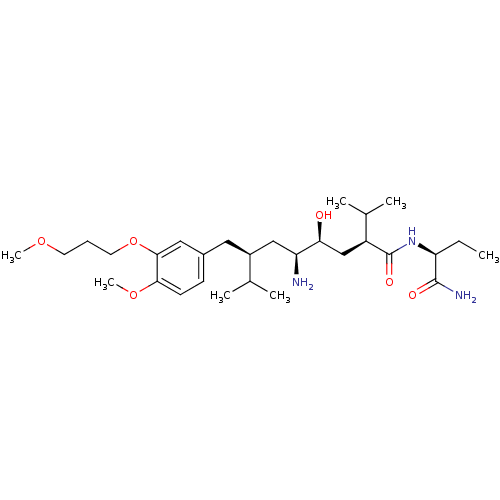 ((2S,4S,5S,7S)-5-amino-N-[(1S)-1-carbamoylpropyl]-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18336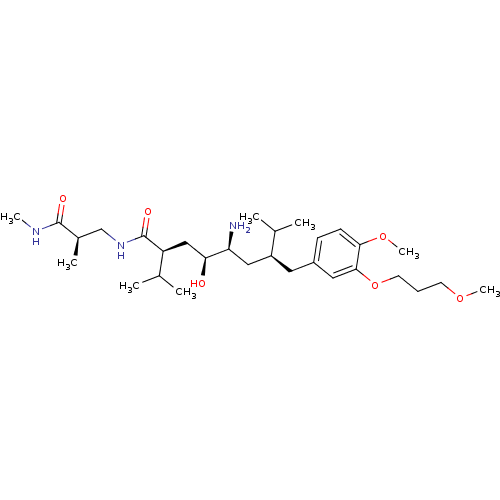 ((2S,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18334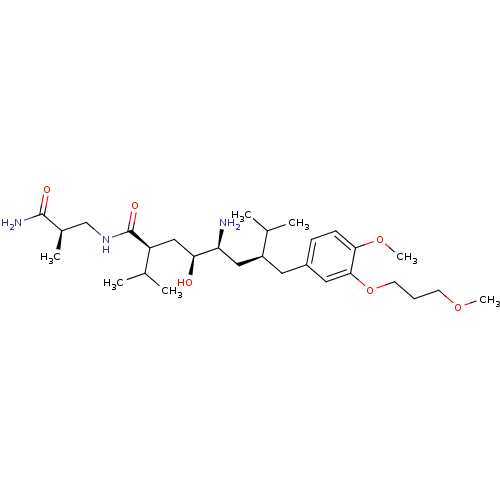 ((2S,4S,5S,7S)-5-amino-N-[(2R)-2-carbamoyl-2-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18335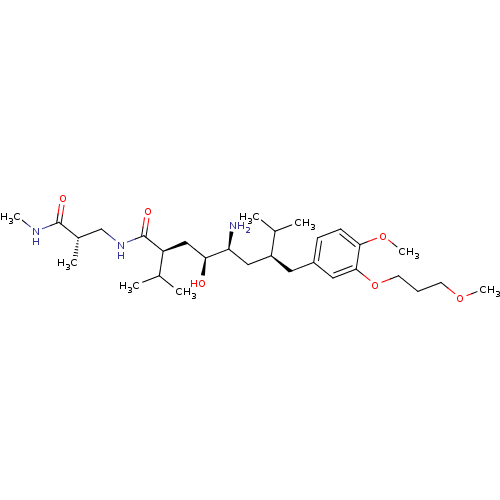 ((2S,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18333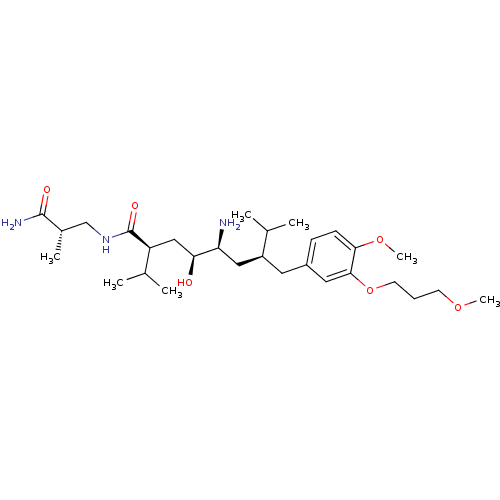 ((2S,4S,5S,7S)-5-amino-N-[(2S)-2-carbamoyl-2-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18331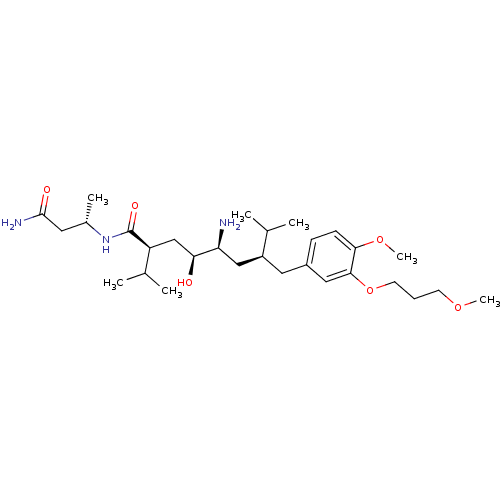 ((2S,4S,5S,7S)-5-amino-N-[(2S)-1-carbamoylpropan-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18350 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18321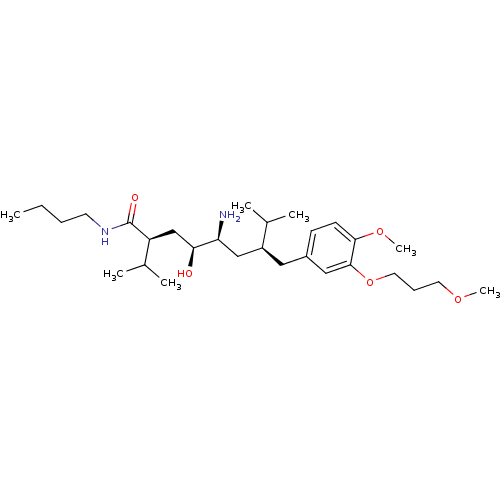 ((2S,4S,5S,7S)-5-amino-N-butyl-4-hydroxy-7-{[4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18322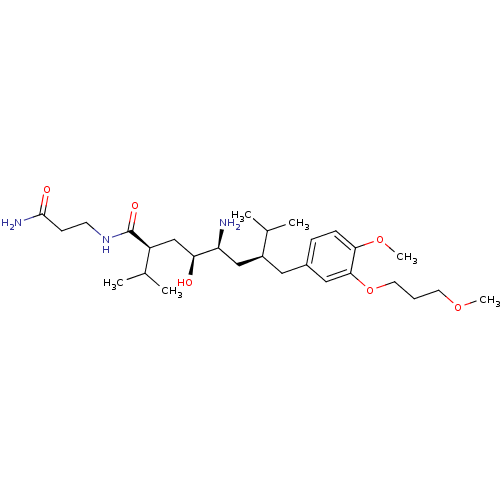 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoylethyl)-4-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18339 ((2S,4S,5S,7S)-5-amino-N-[3-(dimethylcarbamoyl)prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18341 ((2S,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18348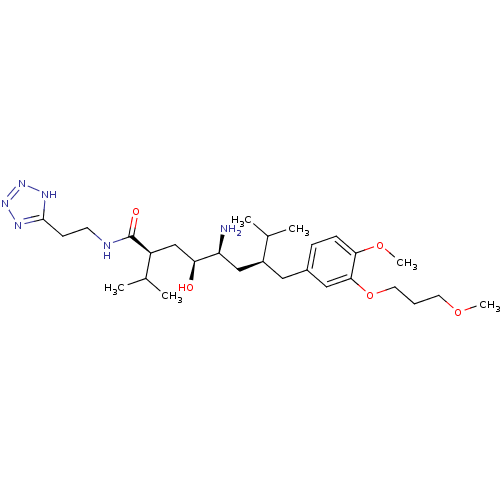 ((2S,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18349 ((2S,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18327 ((2S,4S,5S,7S)-5-amino-N-[(1S)-1-carbamoyl-2-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18329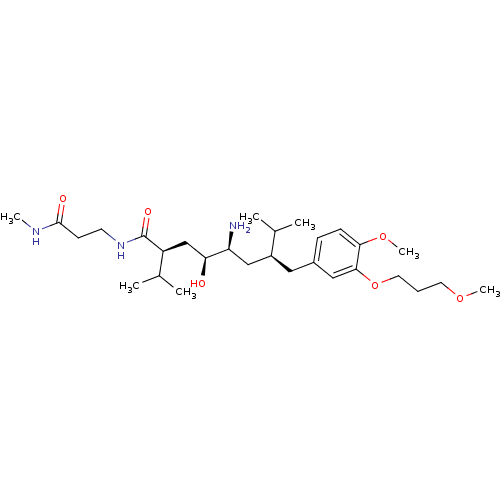 ((2S,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18343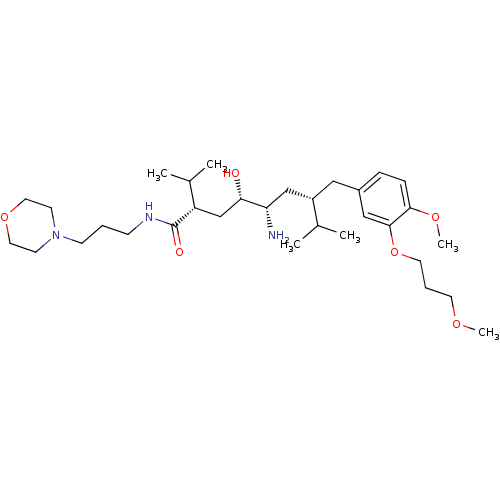 ((2S,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18330 ((2S,4S,5S,7S)-5-amino-N-[2-(dimethylcarbamoyl)ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18347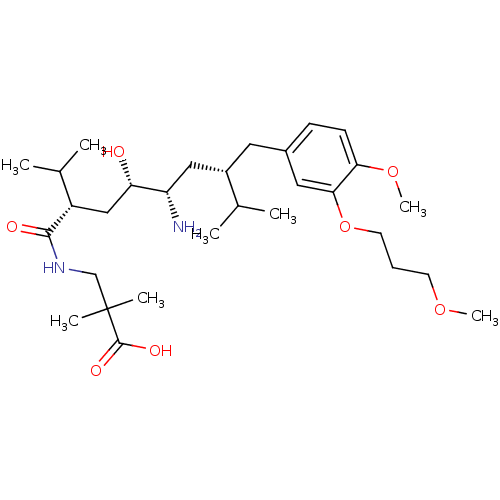 (2,7-dialkyl-substituted 8-phenyl-octanecarboxamide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18346 ((2S,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18324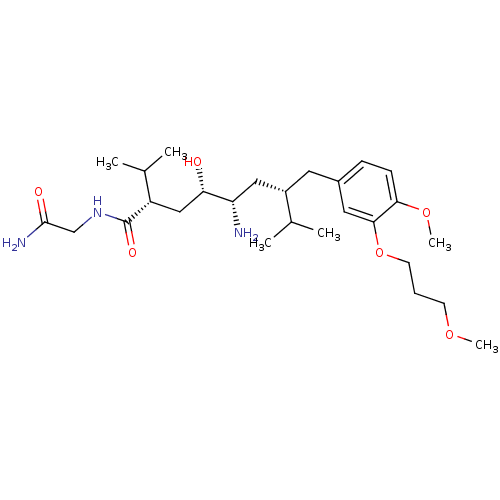 ((2S,4S,5S,7S)-5-amino-N-(carbamoylmethyl)-4-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18325 ((2S,4S,5S,7S)-5-amino-N-[(1S)-1-carbamoylethyl]-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18332 ((2S,4S,5S,7S)-5-amino-N-[(2R)-1-carbamoylpropan-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18328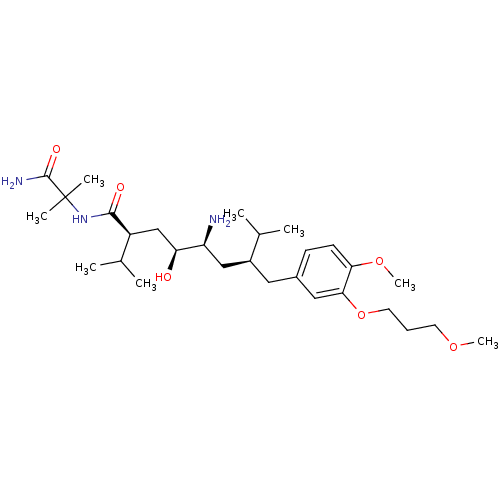 ((2S,4S,5S,7S)-5-amino-N-(1-carbamoyl-1-methylethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18345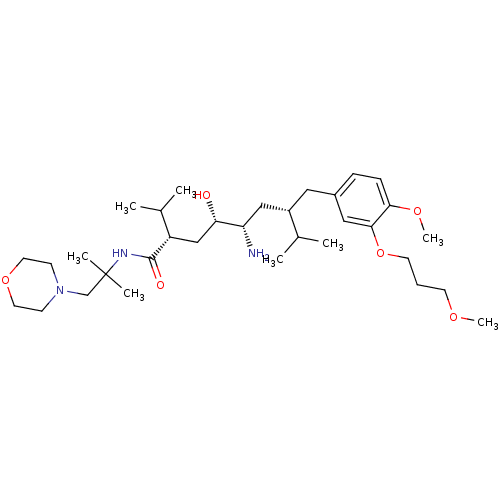 ((2S,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4832-44 (2007) Article DOI: 10.1021/jm070316i BindingDB Entry DOI: 10.7270/Q2GQ6W1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||