

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosylhomocysteinase (Mus musculus) | BDBM50008288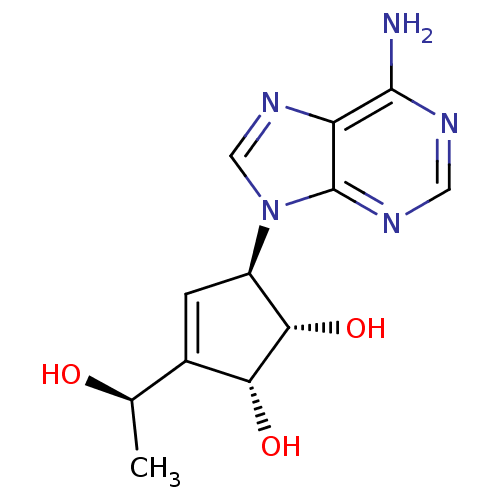 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-((R)-1-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyo Jozo Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect of the compound against L929 Cell S-adenosyl-L-homocysteine hydrolase | J Med Chem 35: 324-31 (1992) BindingDB Entry DOI: 10.7270/Q2RX9B1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50006223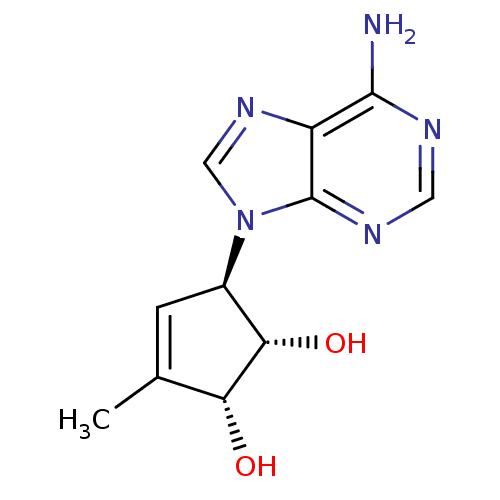 (5-(6-Amino-purin-9-yl)-3-methyl-cyclopent-3-ene-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyo Jozo Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect of the compound against L929 Cell S-adenosyl-L-homocysteine hydrolase | J Med Chem 35: 324-31 (1992) BindingDB Entry DOI: 10.7270/Q2RX9B1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50008289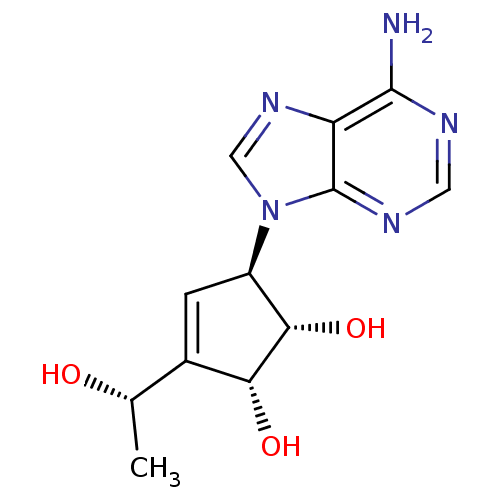 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-((S)-1-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyo Jozo Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect of the compound against L929 Cell S-adenosyl-L-homocysteine hydrolase | J Med Chem 35: 324-31 (1992) BindingDB Entry DOI: 10.7270/Q2RX9B1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006222 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 15.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyo Jozo Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect against S-adenosyl-L-homocysteine hydrolase of rabbit erythrocyte. | J Med Chem 35: 324-31 (1992) BindingDB Entry DOI: 10.7270/Q2RX9B1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50008288 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-((R)-1-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 721 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyo Jozo Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect against S-adenosyl-L-homocysteine hydrolase of rabbit erythrocyte. | J Med Chem 35: 324-31 (1992) BindingDB Entry DOI: 10.7270/Q2RX9B1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50230014 (CHEMBL320262) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyo Jozo Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect against S-adenosyl-L-homocysteine hydrolase of rabbit erythrocyte. | J Med Chem 35: 324-31 (1992) BindingDB Entry DOI: 10.7270/Q2RX9B1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50018500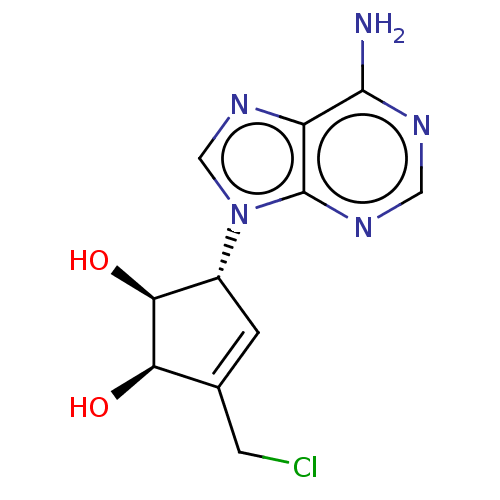 (CHEMBL419393) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyo Jozo Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect against S-adenosyl-L-homocysteine hydrolase of rabbit erythrocyte. | J Med Chem 35: 324-31 (1992) BindingDB Entry DOI: 10.7270/Q2RX9B1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50230012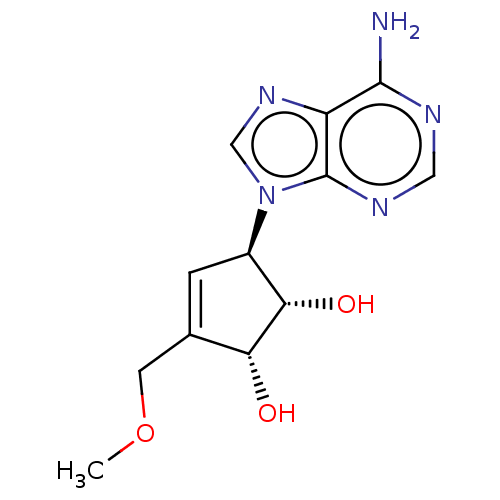 (CHEMBL325316) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyo Jozo Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect against S-adenosyl-L-homocysteine hydrolase of rabbit erythrocyte. | J Med Chem 35: 324-31 (1992) BindingDB Entry DOI: 10.7270/Q2RX9B1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50008289 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-((S)-1-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyo Jozo Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect against S-adenosyl-L-homocysteine hydrolase of rabbit erythrocyte. | J Med Chem 35: 324-31 (1992) BindingDB Entry DOI: 10.7270/Q2RX9B1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50230013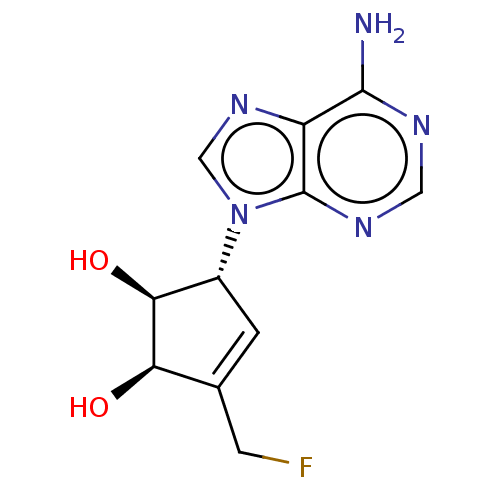 (CHEMBL109444) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyo Jozo Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect against S-adenosyl-L-homocysteine hydrolase of rabbit erythrocyte. | J Med Chem 35: 324-31 (1992) BindingDB Entry DOI: 10.7270/Q2RX9B1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50230015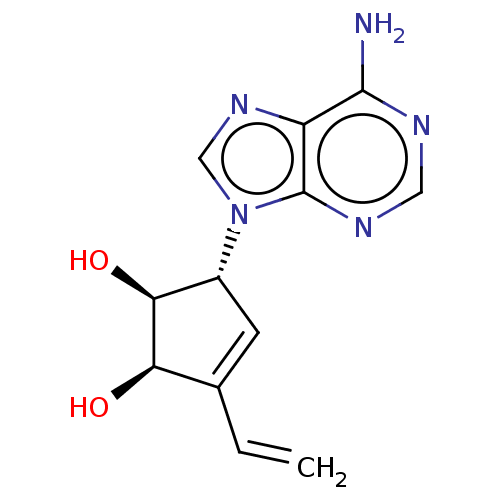 (CHEMBL324105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyo Jozo Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect against S-adenosyl-L-homocysteine hydrolase of rabbit erythrocyte. | J Med Chem 35: 324-31 (1992) BindingDB Entry DOI: 10.7270/Q2RX9B1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006223 (5-(6-Amino-purin-9-yl)-3-methyl-cyclopent-3-ene-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >4.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyo Jozo Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect against S-adenosyl-L-homocysteine hydrolase of rabbit erythrocyte. | J Med Chem 35: 324-31 (1992) BindingDB Entry DOI: 10.7270/Q2RX9B1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||