

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity towards progesterone receptor was measured | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50066523 (2,2,4-Trimethyl-6-(3-nitro-phenyl)-1,2-dihydro-qui...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity towards progesterone receptor was measured | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105572 (1-Benzyl-6-(3-nitro-phenyl)-1,3-dihydro-benzoimida...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity towards progesterone receptor was measured | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105564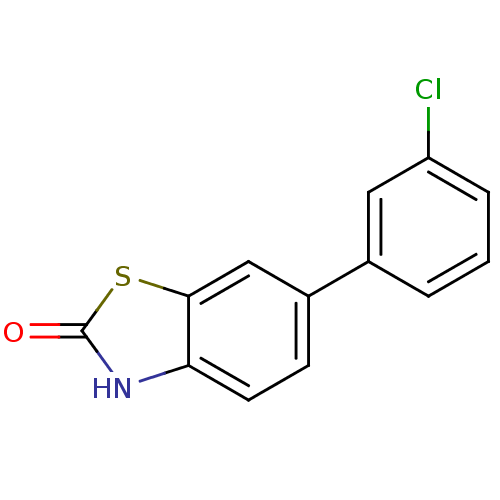 (6-(3-Chloro-phenyl)-3H-benzothiazol-2-one | CHEMBL...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone stimulated alkaline phosphatase activity in T47D human breast carcinoma cell line | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105561 (1-Benzyl-6-(3-chloro-phenyl)-1,3-dihydro-benzoimid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity towards progesterone receptor was measured | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105566 (6-(3-Nitro-phenyl)-3H-benzothiazol-2-one | CHEMBL3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 332 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity towards progesterone receptor was measured | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105563 (1-Benzyl-6-(3-chloro-phenyl)-1,3-dihydro-benzoimid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 521 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity towards progesterone receptor was measured | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105565 (6-(3-Chloro-phenyl)-1-isopropyl-1,3-dihydro-benzoi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 525 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity towards progesterone receptor was measured | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105560 (1-Isopropyl-6-(3-nitro-phenyl)-1,3-dihydro-benzoim...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 525 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity towards progesterone receptor was measured | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105562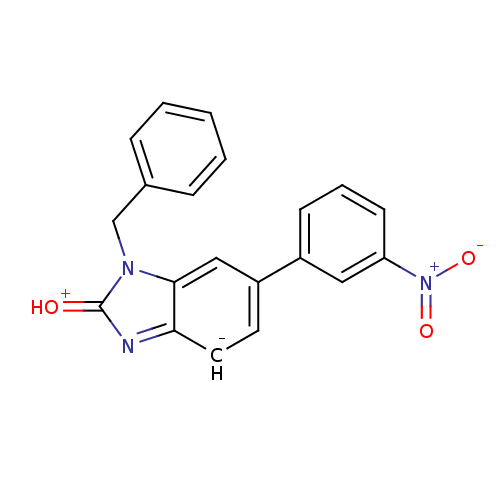 (1-Benzyl-6-(3-nitro-phenyl)-1,3-dihydro-benzoimida...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 714 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity towards progesterone receptor was measured | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105559 (1-Benzyl-5-(3-chloro-phenyl)-1,3-dihydro-benzoimid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity towards progesterone receptor was measured | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105568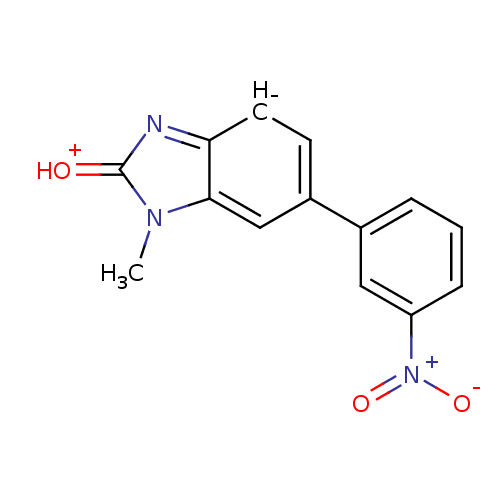 (1-Methyl-6-(3-nitro-phenyl)-1,3-dihydro-benzoimida...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity towards progesterone receptor was measured | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105567 (1-Benzyl-5-(3-nitro-phenyl)-1,3-dihydro-benzoimida...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone stimulated alkaline phosphatase activity in T47D human breast carcinoma cell line | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone stimulated alkaline phosphatase activity in T47D human breast carcinoma cell line | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone induced PRE-luciferase activity in CV-cells | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105572 (1-Benzyl-6-(3-nitro-phenyl)-1,3-dihydro-benzoimida...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone stimulated alkaline phosphatase activity in T47D human breast carcinoma cell line | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105561 (1-Benzyl-6-(3-chloro-phenyl)-1,3-dihydro-benzoimid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone stimulated alkaline phosphatase activity in T47D human breast carcinoma cell line | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105571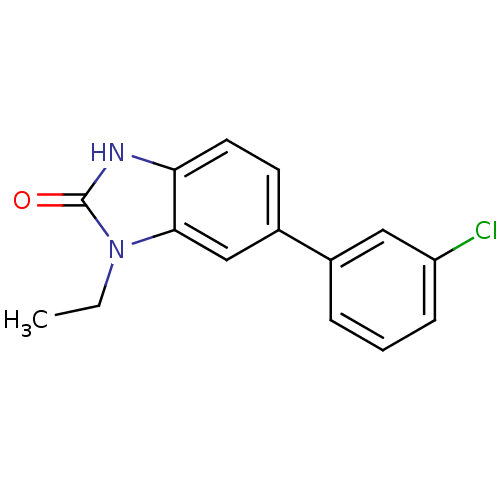 (6-(3-Chloro-phenyl)-1-ethyl-1,3-dihydro-benzoimida...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone stimulated alkaline phosphatase activity in T47D human breast carcinoma cell line | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50066523 (2,2,4-Trimethyl-6-(3-nitro-phenyl)-1,2-dihydro-qui...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone stimulated alkaline phosphatase activity in T47D human breast carcinoma cell line | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50066523 (2,2,4-Trimethyl-6-(3-nitro-phenyl)-1,2-dihydro-qui...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity towards progesterone receptor was measured | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105562 (1-Benzyl-6-(3-nitro-phenyl)-1,3-dihydro-benzoimida...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone stimulated alkaline phosphatase activity in T47D human breast carcinoma cell line | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105560 (1-Isopropyl-6-(3-nitro-phenyl)-1,3-dihydro-benzoim...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone stimulated alkaline phosphatase activity in T47D human breast carcinoma cell line | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105566 (6-(3-Nitro-phenyl)-3H-benzothiazol-2-one | CHEMBL3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity towards progesterone receptor was measured | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105571 (6-(3-Chloro-phenyl)-1-ethyl-1,3-dihydro-benzoimida...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone induced PRE-luciferase activity in CV-cells | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105565 (6-(3-Chloro-phenyl)-1-isopropyl-1,3-dihydro-benzoi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone stimulated alkaline phosphatase activity in T47D human breast carcinoma cell line | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105563 (1-Benzyl-6-(3-chloro-phenyl)-1,3-dihydro-benzoimid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone stimulated alkaline phosphatase activity in T47D human breast carcinoma cell line | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105560 (1-Isopropyl-6-(3-nitro-phenyl)-1,3-dihydro-benzoim...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone induced PRE-luciferase activity in CV-cells | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105562 (1-Benzyl-6-(3-nitro-phenyl)-1,3-dihydro-benzoimida...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 257 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity towards progesterone receptor was measured | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105564 (6-(3-Chloro-phenyl)-3H-benzothiazol-2-one | CHEMBL...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 265 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone induced PRE-luciferase activity in CV-cells | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105566 (6-(3-Nitro-phenyl)-3H-benzothiazol-2-one | CHEMBL3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 326 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone induced PRE-luciferase activity in CV-cells | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105565 (6-(3-Chloro-phenyl)-1-isopropyl-1,3-dihydro-benzoi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 412 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone induced PRE-luciferase activity in CV-cells | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105563 (1-Benzyl-6-(3-chloro-phenyl)-1,3-dihydro-benzoimid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 412 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone induced PRE-luciferase activity in CV-cells | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105564 (6-(3-Chloro-phenyl)-3H-benzothiazol-2-one | CHEMBL...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone stimulated alkaline phosphatase activity in T47D human breast carcinoma cell line | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105570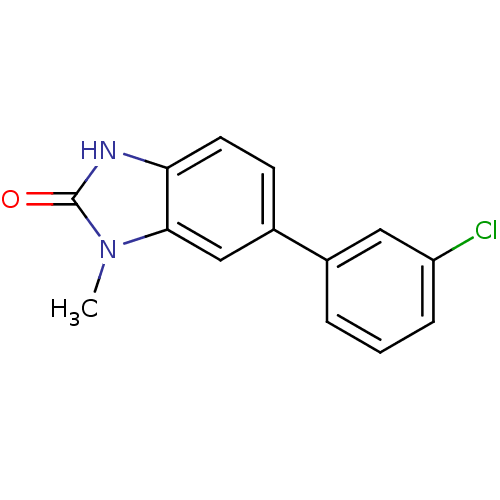 (6-(3-Chloro-phenyl)-1-methyl-1,3-dihydro-benzoimid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone stimulated alkaline phosphatase activity in T47D human breast carcinoma cell line | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105568 (1-Methyl-6-(3-nitro-phenyl)-1,3-dihydro-benzoimida...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone stimulated alkaline phosphatase activity in T47D human breast carcinoma cell line | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105570 (6-(3-Chloro-phenyl)-1-methyl-1,3-dihydro-benzoimid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone induced PRE-luciferase activity in CV-cells | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105568 (1-Methyl-6-(3-nitro-phenyl)-1,3-dihydro-benzoimida...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone induced PRE-luciferase activity in CV-cells | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105559 (1-Benzyl-5-(3-chloro-phenyl)-1,3-dihydro-benzoimid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone induced PRE-luciferase activity in CV-cells | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105567 (1-Benzyl-5-(3-nitro-phenyl)-1,3-dihydro-benzoimida...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone stimulated alkaline phosphatase activity in T47D human breast carcinoma cell line | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105567 (1-Benzyl-5-(3-nitro-phenyl)-1,3-dihydro-benzoimida...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity towards progesterone receptor was measured | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105559 (1-Benzyl-5-(3-chloro-phenyl)-1,3-dihydro-benzoimid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone stimulated alkaline phosphatase activity in T47D human breast carcinoma cell line | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50105569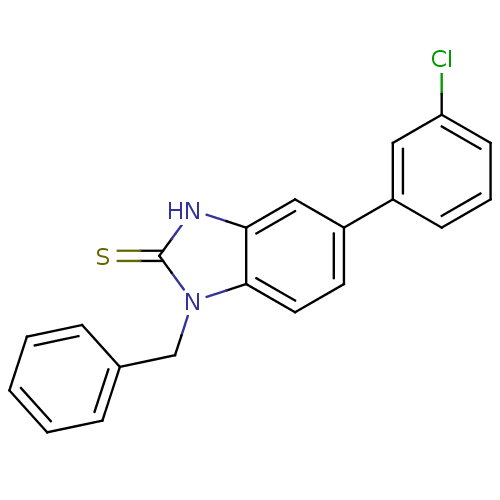 (1-Benzyl-5-(3-chloro-phenyl)-1,3-dihydro-benzoimid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory concentration against progesterone stimulated alkaline phosphatase activity in T47D human breast carcinoma cell line | Bioorg Med Chem Lett 11: 2747-50 (2001) BindingDB Entry DOI: 10.7270/Q2222V92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||