

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neprilysin (Mus musculus) | BDBM50452141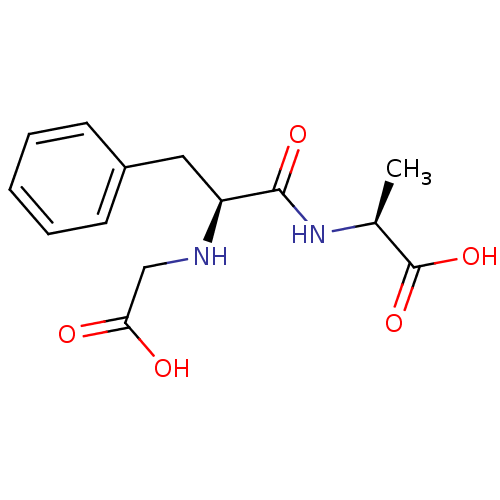 (CHEMBL2372595) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibitory potency against enkephalinase from mouse striatum. | J Med Chem 26: 60-5 (1983) BindingDB Entry DOI: 10.7270/Q2Q81DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Mus musculus) | BDBM50027522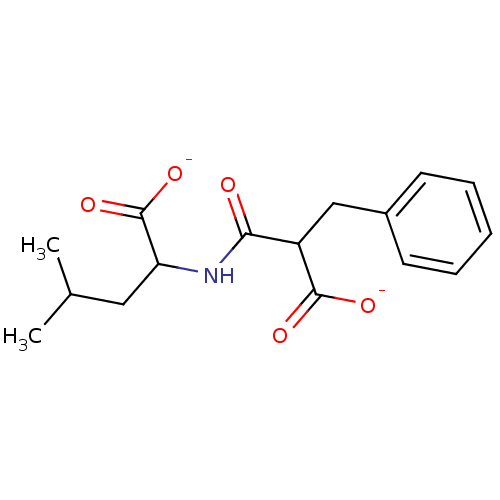 (2-(2-Carboxy-3-phenyl-propionylamino)-4-methyl-pen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibitory potency against enkephalinase from mouse striatum. | J Med Chem 26: 60-5 (1983) BindingDB Entry DOI: 10.7270/Q2Q81DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Mus musculus) | BDBM50027514 (2-(2-Carboxymethyl-3-phenyl-propionylamino)-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibitory potency against enkephalinase from mouse striatum. | J Med Chem 26: 60-5 (1983) BindingDB Entry DOI: 10.7270/Q2Q81DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Mus musculus) | BDBM50139892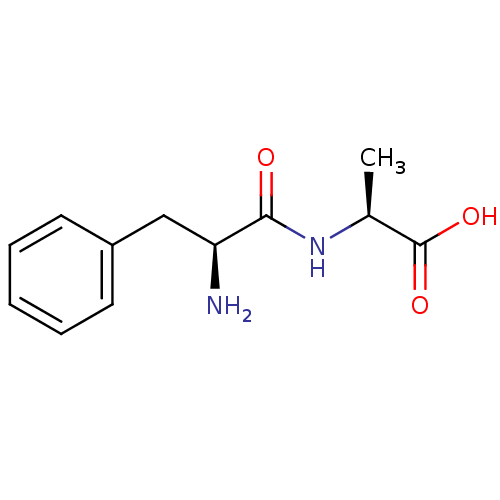 ((S)-2-((S)-2-Amino-3-phenyl-propionylamino)-propio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibitory potency against enkephalinase from mouse striatum. | J Med Chem 26: 60-5 (1983) BindingDB Entry DOI: 10.7270/Q2Q81DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Mus musculus) | BDBM50027518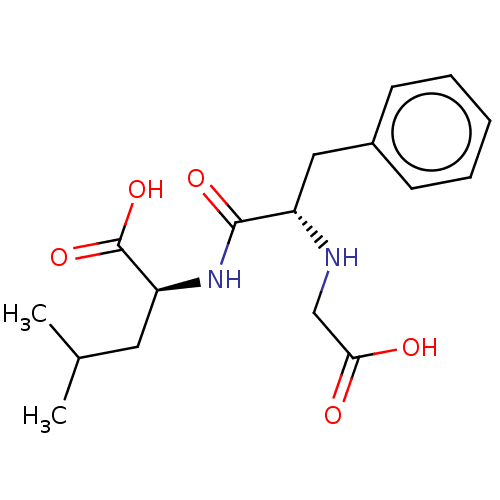 ((2S)-2-[(2R)-2-[(carboxylatomethyl)amino]-3-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibitory potency against enkephalinase from mouse striatum. | J Med Chem 26: 60-5 (1983) BindingDB Entry DOI: 10.7270/Q2Q81DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50027518 ((2S)-2-[(2R)-2-[(carboxylatomethyl)amino]-3-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibitory potency against angiotensin I converting enzyme from mouse striatum. | J Med Chem 26: 60-5 (1983) BindingDB Entry DOI: 10.7270/Q2Q81DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50452141 (CHEMBL2372595) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibitory potency against angiotensin I converting enzyme from mouse striatum. | J Med Chem 26: 60-5 (1983) BindingDB Entry DOI: 10.7270/Q2Q81DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Mus musculus) | BDBM50027513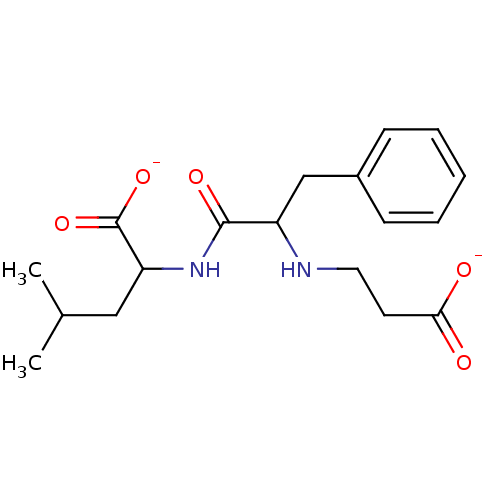 (2-[2-(2-Carboxy-ethylamino)-3-phenyl-propionylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibitory potency against enkephalinase from mouse striatum. | J Med Chem 26: 60-5 (1983) BindingDB Entry DOI: 10.7270/Q2Q81DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Mus musculus) | BDBM50224343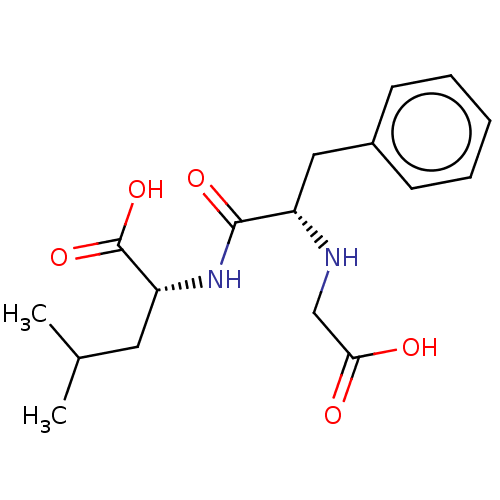 (CHEMBL3350383) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibitory potency against enkephalinase from mouse striatum. | J Med Chem 26: 60-5 (1983) BindingDB Entry DOI: 10.7270/Q2Q81DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Mus musculus) | BDBM50027517 (CHEMBL2372594) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibitory potency against enkephalinase from mouse striatum. | J Med Chem 26: 60-5 (1983) BindingDB Entry DOI: 10.7270/Q2Q81DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50027514 (2-(2-Carboxymethyl-3-phenyl-propionylamino)-4-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibitory potency against angiotensin I converting enzyme from mouse striatum. | J Med Chem 26: 60-5 (1983) BindingDB Entry DOI: 10.7270/Q2Q81DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50027513 (2-[2-(2-Carboxy-ethylamino)-3-phenyl-propionylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibitory potency against angiotensin I converting enzyme from mouse striatum. | J Med Chem 26: 60-5 (1983) BindingDB Entry DOI: 10.7270/Q2Q81DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Mus musculus) | BDBM50027518 ((2S)-2-[(2R)-2-[(carboxylatomethyl)amino]-3-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibitory potency against enkephalinase from mouse striatum. | J Med Chem 26: 60-5 (1983) BindingDB Entry DOI: 10.7270/Q2Q81DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Mus musculus) | BDBM50027516 (2-(2-Amino-3-phenyl-propionylamino)-4-methyl-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibitory potency against enkephalinase from mouse striatum. | J Med Chem 26: 60-5 (1983) BindingDB Entry DOI: 10.7270/Q2Q81DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50027516 (2-(2-Amino-3-phenyl-propionylamino)-4-methyl-penta...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibitory potency against Angiotensin I converting enzyme from mouse striatum. | J Med Chem 26: 60-5 (1983) BindingDB Entry DOI: 10.7270/Q2Q81DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50027522 (2-(2-Carboxy-3-phenyl-propionylamino)-4-methyl-pen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibitory potency against Angiotensin I converting enzyme from mouse striatum. | J Med Chem 26: 60-5 (1983) BindingDB Entry DOI: 10.7270/Q2Q81DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50139892 ((S)-2-((S)-2-Amino-3-phenyl-propionylamino)-propio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibitory potency against Angiotensin I converting enzyme from mouse striatum. | J Med Chem 26: 60-5 (1983) BindingDB Entry DOI: 10.7270/Q2Q81DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||