

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50023370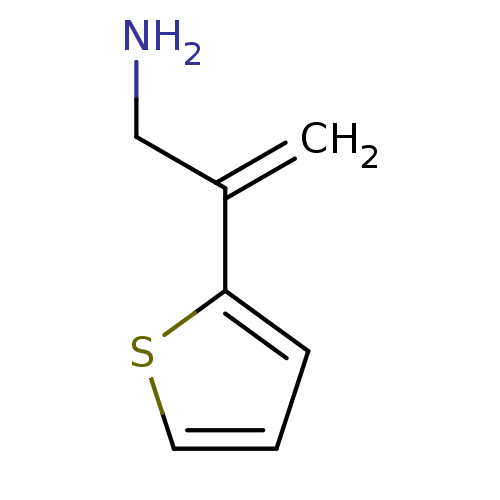 (2-Thiophen-2-yl-allylamine | CHEMBL110604) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description KI value was determined from plots of 1/kinact(observed) vs 1/[inhibitor] | J Med Chem 31: 704-6 (1988) BindingDB Entry DOI: 10.7270/Q26972KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50017834 (4-(1-Aminomethyl-prop-2-ynyl)-phenol | CHEMBL16312...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description KI value was determined from plots of 1/kinact(observed) vs 1/[inhibitor] | J Med Chem 31: 704-6 (1988) BindingDB Entry DOI: 10.7270/Q26972KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50017834 (4-(1-Aminomethyl-prop-2-ynyl)-phenol | CHEMBL16312...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description KI value was determined from plots of 1/kinact(observed) vs 1/[inhibitor] | J Med Chem 31: 704-6 (1988) BindingDB Entry DOI: 10.7270/Q26972KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50017827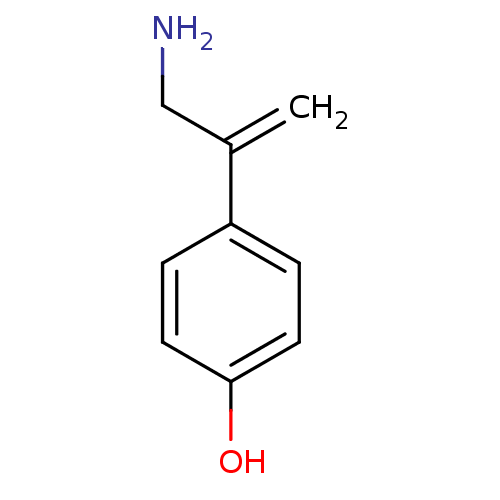 (4-(1-Aminomethyl-vinyl)-phenol | CHEMBL330118) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description KI value was determined from plots of 1/kinact(observed) vs 1/[inhibitor]; Apparent values at pH 5.0, 0.24 mM O2 | J Med Chem 31: 704-6 (1988) BindingDB Entry DOI: 10.7270/Q26972KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50023371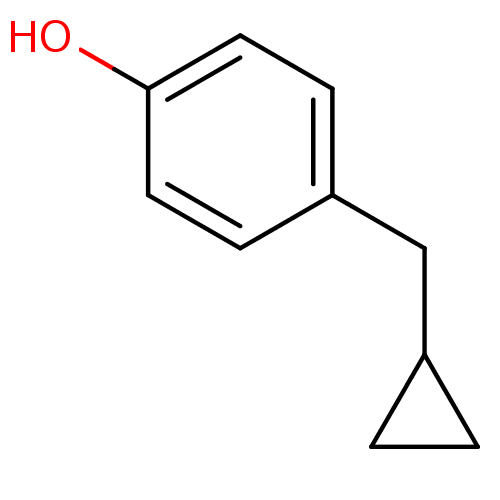 (4-Cyclopropylmethyl-phenol | CHEMBL162030) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description KI value was determined from plots of 1/kinact(observed) vs 1/[inhibitor] | J Med Chem 31: 704-6 (1988) BindingDB Entry DOI: 10.7270/Q26972KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||