 Found 35 hits Enz. Inhib. hit(s) with all data for entry = 50005028
Found 35 hits Enz. Inhib. hit(s) with all data for entry = 50005028 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031677

((S)-13-Methyl-17-pyridin-3-yl-7,8,9,11,12,13,14,15...)Show SMILES C[C@]12CCC3C(CCc4cc(O)ccc34)C1CC=C2c1cccnc1 |c:20| Show InChI InChI=1S/C23H25NO/c1-23-11-10-19-18-7-5-17(25)13-15(18)4-6-20(19)22(23)9-8-21(23)16-3-2-12-24-14-16/h2-3,5,7-8,12-14,19-20,22,25H,4,6,9-11H2,1H3/t19?,20?,22?,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031666
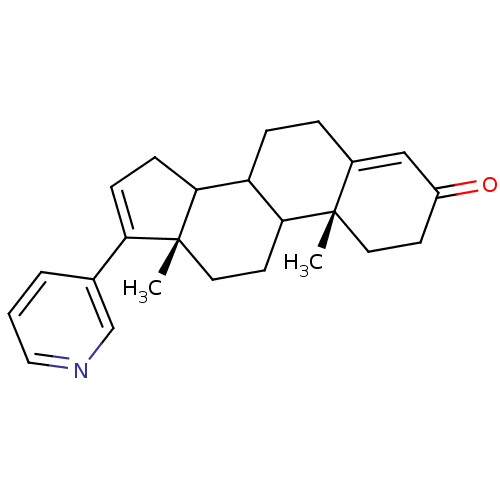
((10R,13S)-10,13-Dimethyl-17-pyridin-3-yl-1,2,6,7,8...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2c1cccnc1 |c:21,t:8| Show InChI InChI=1S/C24H29NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-4,7,13-15,19,21-22H,5-6,8-12H2,1-2H3/t19?,21?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031669

((3R,5S,10S,13S)-10,13-Dimethyl-17-pyridin-3-yl-2,3...)Show SMILES C[C@]12CCC3C(CC[C@H]4C[C@H](O)CC[C@]34C)C1CC=C2c1cccnc1 |c:21| Show InChI InChI=1S/C24H33NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-4,7,13,15,17-19,21-22,26H,5-6,8-12,14H2,1-2H3/t17-,18+,19?,21?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031677

((S)-13-Methyl-17-pyridin-3-yl-7,8,9,11,12,13,14,15...)Show SMILES C[C@]12CCC3C(CCc4cc(O)ccc34)C1CC=C2c1cccnc1 |c:20| Show InChI InChI=1S/C23H25NO/c1-23-11-10-19-18-7-5-17(25)13-15(18)4-6-20(19)22(23)9-8-21(23)16-3-2-12-24-14-16/h2-3,5,7-8,12-14,19-20,22,25H,4,6,9-11H2,1H3/t19?,20?,22?,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031666

((10R,13S)-10,13-Dimethyl-17-pyridin-3-yl-1,2,6,7,8...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2c1cccnc1 |c:21,t:8| Show InChI InChI=1S/C24H29NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-4,7,13-15,19,21-22H,5-6,8-12H2,1-2H3/t19?,21?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031665
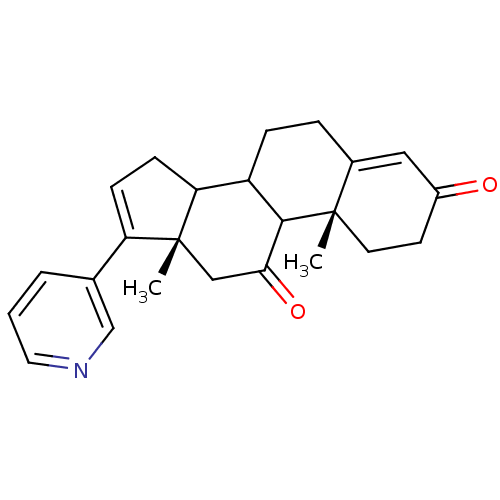
((10R,13S)-10,13-Dimethyl-17-pyridin-3-yl-1,6,7,8,9...)Show SMILES C[C@]12CC(=O)C3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2c1cccnc1 |c:22,t:9| Show InChI InChI=1S/C24H27NO2/c1-23-10-9-17(26)12-16(23)5-6-18-20-8-7-19(15-4-3-11-25-14-15)24(20,2)13-21(27)22(18)23/h3-4,7,11-12,14,18,20,22H,5-6,8-10,13H2,1-2H3/t18?,20?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM25458

((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...)Show SMILES [H][C@@]12CC=C(c3cccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |t:3,23| Show InChI InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-5,7,13,15,18-19,21-22,26H,6,8-12,14H2,1-2H3/t18-,19-,21-,22-,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031676
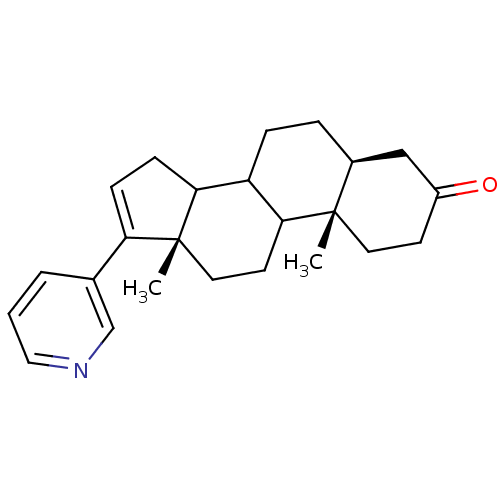
((5S,10S,13S)-10,13-Dimethyl-17-pyridin-3-yl-1,2,4,...)Show SMILES C[C@]12CCC3C(CC[C@H]4CC(=O)CC[C@]34C)C1CC=C2c1cccnc1 |c:21| Show InChI InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-4,7,13,15,17,19,21-22H,5-6,8-12,14H2,1-2H3/t17-,19?,21?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM25458

((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...)Show SMILES [H][C@@]12CC=C(c3cccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |t:3,23| Show InChI InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-5,7,13,15,18-19,21-22,26H,6,8-12,14H2,1-2H3/t18-,19-,21-,22-,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031669

((3R,5S,10S,13S)-10,13-Dimethyl-17-pyridin-3-yl-2,3...)Show SMILES C[C@]12CCC3C(CC[C@H]4C[C@H](O)CC[C@]34C)C1CC=C2c1cccnc1 |c:21| Show InChI InChI=1S/C24H33NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-4,7,13,15,17-19,21-22,26H,5-6,8-12,14H2,1-2H3/t17-,18+,19?,21?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031676

((5S,10S,13S)-10,13-Dimethyl-17-pyridin-3-yl-1,2,4,...)Show SMILES C[C@]12CCC3C(CC[C@H]4CC(=O)CC[C@]34C)C1CC=C2c1cccnc1 |c:21| Show InChI InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-4,7,13,15,17,19,21-22H,5-6,8-12,14H2,1-2H3/t17-,19?,21?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031667

(3-((10R,13S)-10,13-Dimethyl-2,7,8,9,10,11,12,13,14...)Show SMILES C[C@]12CCC3C(CC=C4C=CCC[C@]34C)C1CC=C2c1cccnc1 |c:9,20,t:7| Show InChI InChI=1S/C24H29N/c1-23-13-4-3-7-18(23)8-9-19-21-11-10-20(17-6-5-15-25-16-17)24(21,2)14-12-22(19)23/h3,5-8,10,15-16,19,21-22H,4,9,11-14H2,1-2H3/t19?,21?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031667

(3-((10R,13S)-10,13-Dimethyl-2,7,8,9,10,11,12,13,14...)Show SMILES C[C@]12CCC3C(CC=C4C=CCC[C@]34C)C1CC=C2c1cccnc1 |c:9,20,t:7| Show InChI InChI=1S/C24H29N/c1-23-13-4-3-7-18(23)8-9-19-21-11-10-20(17-6-5-15-25-16-17)24(21,2)14-12-22(19)23/h3,5-8,10,15-16,19,21-22H,4,9,11-14H2,1-2H3/t19?,21?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031665

((10R,13S)-10,13-Dimethyl-17-pyridin-3-yl-1,6,7,8,9...)Show SMILES C[C@]12CC(=O)C3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2c1cccnc1 |c:22,t:9| Show InChI InChI=1S/C24H27NO2/c1-23-10-9-17(26)12-16(23)5-6-18-20-8-7-19(15-4-3-11-25-14-15)24(20,2)13-21(27)22(18)23/h3-4,7,11-12,14,18,20,22H,5-6,8-10,13H2,1-2H3/t18?,20?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50407398
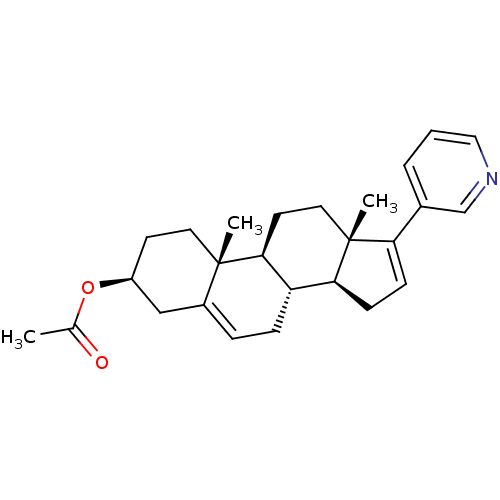
(ABIRATERONE ACETATE | CB7630 | Zytiga)Show SMILES CC(=O)O[C@H]1CC[C@]2(C)[C@H]3CC[C@@]4(C)[C@@H](CC=C4c4cccnc4)[C@@H]3CC=C2C1 |r,c:16,29| Show InChI InChI=1S/C26H33NO2/c1-17(28)29-20-10-12-25(2)19(15-20)6-7-21-23-9-8-22(18-5-4-14-27-16-18)26(23,3)13-11-24(21)25/h4-6,8,14,16,20-21,23-24H,7,9-13,15H2,1-3H3/t20-,21-,23-,24-,25-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50407398

(ABIRATERONE ACETATE | CB7630 | Zytiga)Show SMILES CC(=O)O[C@H]1CC[C@]2(C)[C@H]3CC[C@@]4(C)[C@@H](CC=C4c4cccnc4)[C@@H]3CC=C2C1 |r,c:16,29| Show InChI InChI=1S/C26H33NO2/c1-17(28)29-20-10-12-25(2)19(15-20)6-7-21-23-9-8-22(18-5-4-14-27-16-18)26(23,3)13-11-24(21)25/h4-6,8,14,16,20-21,23-24H,7,9-13,15H2,1-3H3/t20-,21-,23-,24-,25-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031671
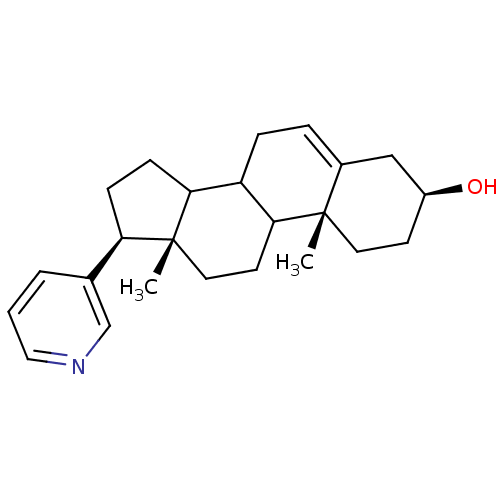
((3S,10R,13S,17S)-10,13-Dimethyl-17-pyridin-3-yl-2,...)Show SMILES C[C@]12CCC3C(CC=C4C[C@@H](O)CC[C@]34C)C1CC[C@@H]2c1cccnc1 |t:7| Show InChI InChI=1S/C24H33NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-5,13,15,18-22,26H,6-12,14H2,1-2H3/t18-,19?,20+,21?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM31768
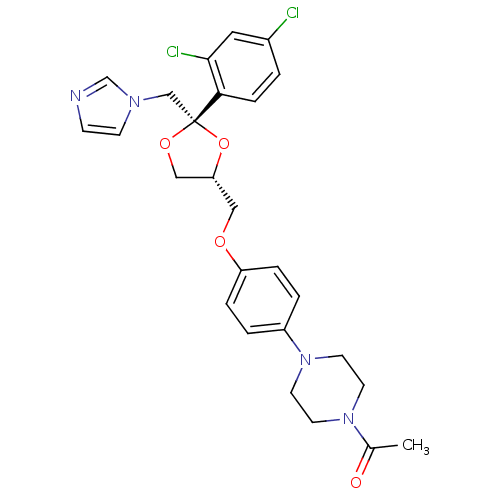
(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031671

((3S,10R,13S,17S)-10,13-Dimethyl-17-pyridin-3-yl-2,...)Show SMILES C[C@]12CCC3C(CC=C4C[C@@H](O)CC[C@]34C)C1CC[C@@H]2c1cccnc1 |t:7| Show InChI InChI=1S/C24H33NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-5,13,15,18-22,26H,6-12,14H2,1-2H3/t18-,19?,20+,21?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031674
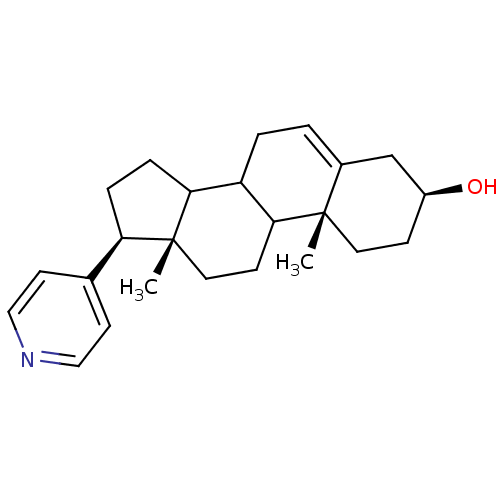
((3S,10R,13S,17R)-10,13-Dimethyl-17-pyridin-4-yl-2,...)Show SMILES C[C@]12CCC3C(CC=C4C[C@@H](O)CC[C@]34C)C1CC[C@@H]2c1ccncc1 |t:7| Show InChI InChI=1S/C24H33NO/c1-23-11-7-18(26)15-17(23)3-4-19-21-6-5-20(16-9-13-25-14-10-16)24(21,2)12-8-22(19)23/h3,9-10,13-14,18-22,26H,4-8,11-12,15H2,1-2H3/t18-,19?,20+,21?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50407397
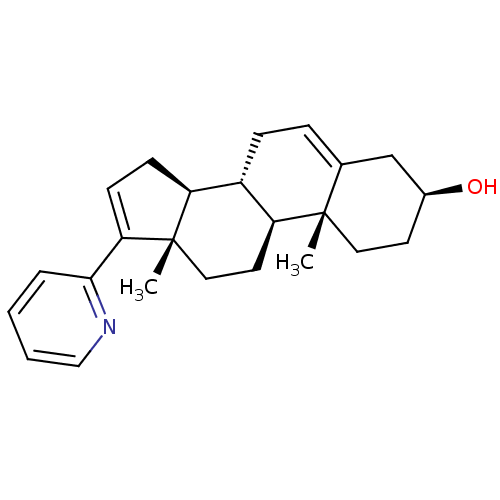
(CHEMBL2112301)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC=C2c1ccccn1 |c:21,t:7| Show InChI InChI=1S/C24H31NO/c1-23-12-10-17(26)15-16(23)6-7-18-19-8-9-21(22-5-3-4-14-25-22)24(19,2)13-11-20(18)23/h3-6,9,14,17-20,26H,7-8,10-13,15H2,1-2H3/t17-,18-,19-,20-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031674

((3S,10R,13S,17R)-10,13-Dimethyl-17-pyridin-4-yl-2,...)Show SMILES C[C@]12CCC3C(CC=C4C[C@@H](O)CC[C@]34C)C1CC[C@@H]2c1ccncc1 |t:7| Show InChI InChI=1S/C24H33NO/c1-23-11-7-18(26)15-17(23)3-4-19-21-6-5-20(16-9-13-25-14-10-16)24(21,2)12-8-22(19)23/h3,9-10,13-14,18-22,26H,4-8,11-12,15H2,1-2H3/t18-,19?,20+,21?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50407397

(CHEMBL2112301)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC=C2c1ccccn1 |c:21,t:7| Show InChI InChI=1S/C24H31NO/c1-23-12-10-17(26)15-16(23)6-7-18-19-8-9-21(22-5-3-4-14-25-22)24(19,2)13-11-20(18)23/h3-6,9,14,17-20,26H,7-8,10-13,15H2,1-2H3/t17-,18-,19-,20-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50407396
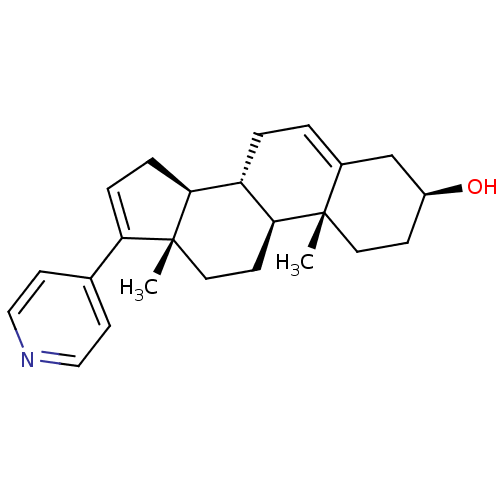
(CHEMBL2112299)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC=C2c1ccncc1 |c:21,t:7| Show InChI InChI=1S/C24H31NO/c1-23-11-7-18(26)15-17(23)3-4-19-21-6-5-20(16-9-13-25-14-10-16)24(21,2)12-8-22(19)23/h3,5,9-10,13-14,18-19,21-22,26H,4,6-8,11-12,15H2,1-2H3/t18-,19-,21-,22-,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50031666

((10R,13S)-10,13-Dimethyl-17-pyridin-3-yl-1,2,6,7,8...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2c1cccnc1 |c:21,t:8| Show InChI InChI=1S/C24H29NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-4,7,13-15,19,21-22H,5-6,8-12H2,1-2H3/t19?,21?,22?,23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of Cytochrome P450 19A1 |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50407396

(CHEMBL2112299)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC=C2c1ccncc1 |c:21,t:7| Show InChI InChI=1S/C24H31NO/c1-23-11-7-18(26)15-17(23)3-4-19-21-6-5-20(16-9-13-25-14-10-16)24(21,2)12-8-22(19)23/h3,5,9-10,13-14,18-19,21-22,26H,4,6-8,11-12,15H2,1-2H3/t18-,19-,21-,22-,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031675
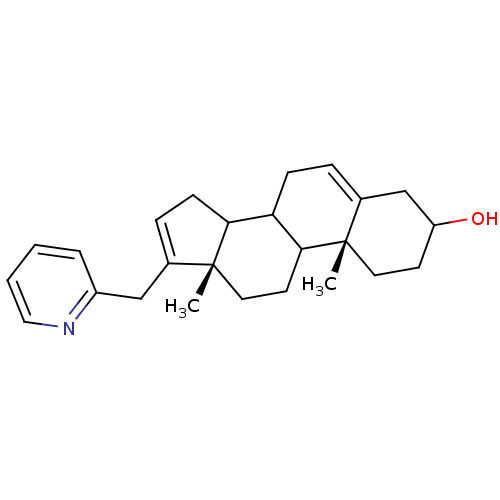
((10R,13S)-10,13-Dimethyl-17-pyridin-2-ylmethyl-2,3...)Show SMILES C[C@]12CCC3C(CC=C4CC(O)CC[C@]34C)C1CC=C2Cc1ccccn1 |c:21,t:7| Show InChI InChI=1S/C25H33NO/c1-24-12-10-20(27)16-18(24)6-8-21-22-9-7-17(15-19-5-3-4-14-26-19)25(22,2)13-11-23(21)24/h3-7,14,20-23,27H,8-13,15-16H2,1-2H3/t20?,21?,22?,23?,24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031672
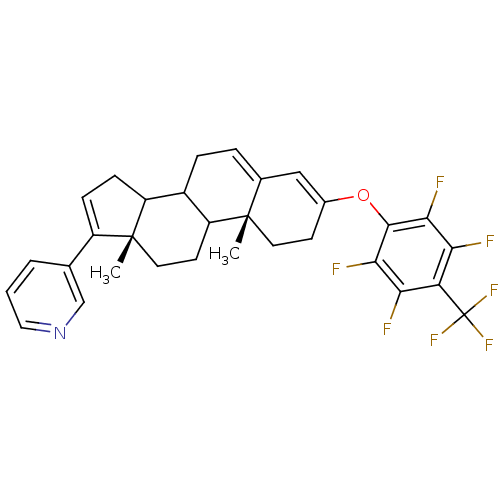
(3-[(10R,13S)-10,13-Dimethyl-3-(2,3,5,6-tetrafluoro...)Show SMILES C[C@]12CCC3C(CC=C4C=C(CC[C@]34C)Oc3c(F)c(F)c(c(F)c3F)C(F)(F)F)C1CC=C2c1cccnc1 |c:9,36,t:7| Show InChI InChI=1S/C31H28F7NO/c1-29-11-9-18(40-28-26(34)24(32)23(31(36,37)38)25(33)27(28)35)14-17(29)5-6-19-21-8-7-20(16-4-3-13-39-15-16)30(21,2)12-10-22(19)29/h3-5,7,13-15,19,21-22H,6,8-12H2,1-2H3/t19?,21?,22?,29-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031672

(3-[(10R,13S)-10,13-Dimethyl-3-(2,3,5,6-tetrafluoro...)Show SMILES C[C@]12CCC3C(CC=C4C=C(CC[C@]34C)Oc3c(F)c(F)c(c(F)c3F)C(F)(F)F)C1CC=C2c1cccnc1 |c:9,36,t:7| Show InChI InChI=1S/C31H28F7NO/c1-29-11-9-18(40-28-26(34)24(32)23(31(36,37)38)25(33)27(28)35)14-17(29)5-6-19-21-8-7-20(16-4-3-13-39-15-16)30(21,2)12-10-22(19)29/h3-5,7,13-15,19,21-22H,6,8-12H2,1-2H3/t19?,21?,22?,29-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031675

((10R,13S)-10,13-Dimethyl-17-pyridin-2-ylmethyl-2,3...)Show SMILES C[C@]12CCC3C(CC=C4CC(O)CC[C@]34C)C1CC=C2Cc1ccccn1 |c:21,t:7| Show InChI InChI=1S/C25H33NO/c1-24-12-10-20(27)16-18(24)6-8-21-22-9-7-17(15-19-5-3-4-14-26-19)25(22,2)13-11-23(21)24/h3-7,14,20-23,27H,8-13,15-16H2,1-2H3/t20?,21?,22?,23?,24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50407397

(CHEMBL2112301)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC=C2c1ccccn1 |c:21,t:7| Show InChI InChI=1S/C24H31NO/c1-23-12-10-17(26)15-16(23)6-7-18-19-8-9-21(22-5-3-4-14-25-22)24(19,2)13-11-20(18)23/h3-6,9,14,17-20,26H,7-8,10-13,15H2,1-2H3/t17-,18-,19-,20-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of Cytochrome P450 19A1 |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50407396

(CHEMBL2112299)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC=C2c1ccncc1 |c:21,t:7| Show InChI InChI=1S/C24H31NO/c1-23-11-7-18(26)15-17(23)3-4-19-21-6-5-20(16-9-13-25-14-10-16)24(21,2)12-8-22(19)23/h3,5,9-10,13-14,18-19,21-22,26H,4,6-8,11-12,15H2,1-2H3/t18-,19-,21-,22-,23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of Cytochrome P450 19A1 |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM25458

((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...)Show SMILES [H][C@@]12CC=C(c3cccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |t:3,23| Show InChI InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-5,7,13,15,18-19,21-22,26H,6,8-12,14H2,1-2H3/t18-,19-,21-,22-,23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of Cytochrome P450 19A1 |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50031676

((5S,10S,13S)-10,13-Dimethyl-17-pyridin-3-yl-1,2,4,...)Show SMILES C[C@]12CCC3C(CC[C@H]4CC(=O)CC[C@]34C)C1CC=C2c1cccnc1 |c:21| Show InChI InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-4,7,13,15,17,19,21-22H,5-6,8-12,14H2,1-2H3/t17-,19?,21?,22?,23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of Cytochrome P450 19A1 |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data