

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50267362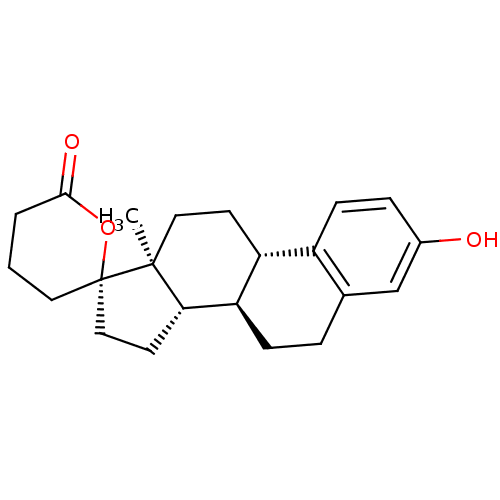 ((2'S,8R,9S,13S,14S)-3-hydroxy-13-methyl-4',5',6,7,...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 (unknown origin) | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358116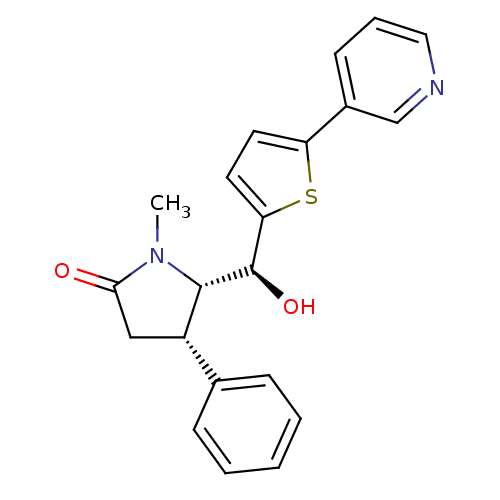 (CHEMBL1915968) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 (unknown origin) | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426580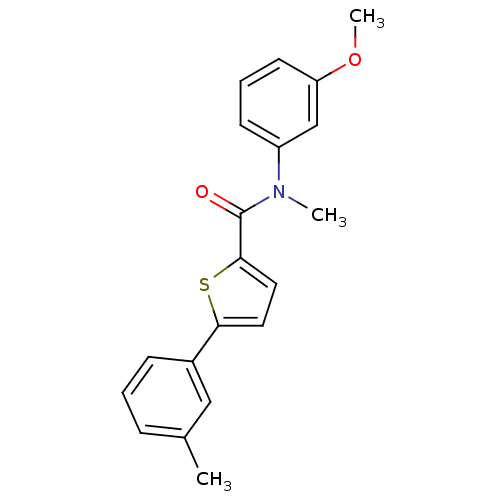 (CHEMBL2324690) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358118 (CHEMBL1915964) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426587 (CHEMBL2324361) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426579 (CHEMBL2324360) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426581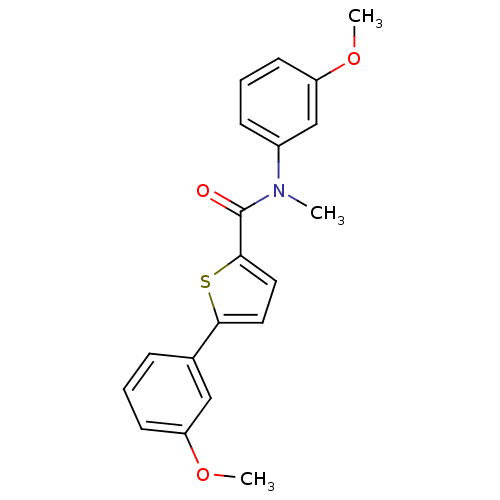 (CHEMBL2324679) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426580 (CHEMBL2324690) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human MDA-MB-231 cells using [3H]E2 as substrate after 6 hrs by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426579 (CHEMBL2324360) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human MDA-MB-231 cells using [3H]E2 as substrate after 6 hrs by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426581 (CHEMBL2324679) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human MDA-MB-231 cells using [3H]E2 as substrate after 6 hrs by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426586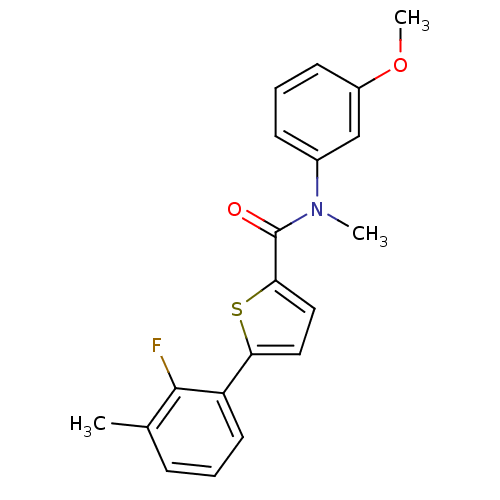 (CHEMBL2324365) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426588 (CHEMBL2324673) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426582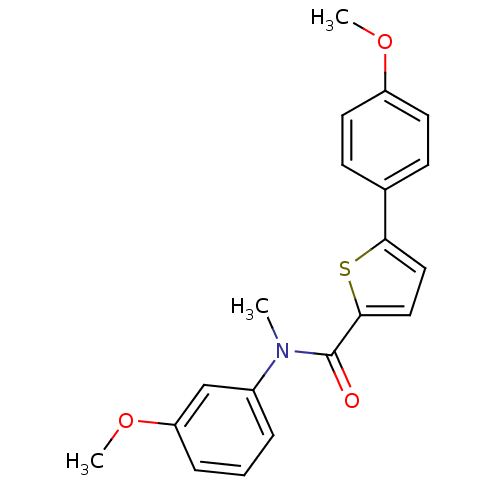 (CHEMBL2324678) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426593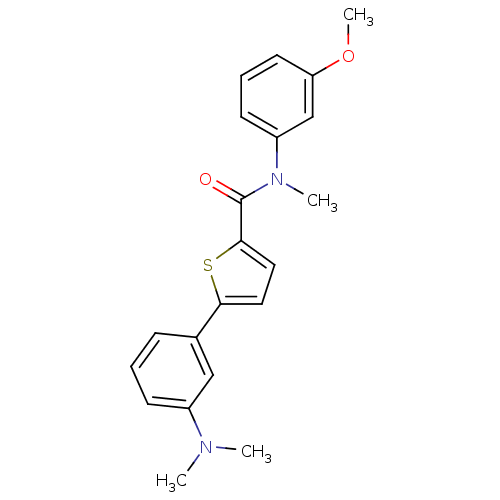 (CHEMBL2324691) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 169 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426585 (CHEMBL2324366) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 184 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426594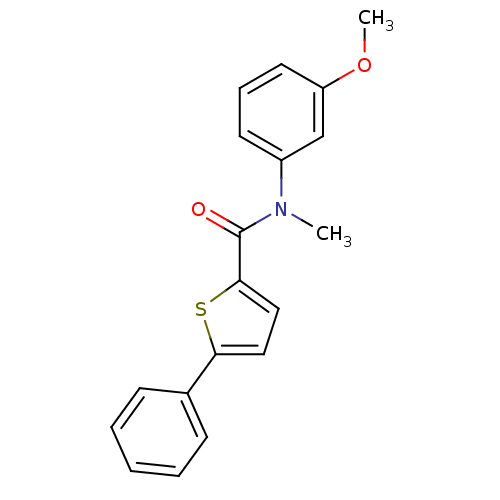 (CHEMBL2324683) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426591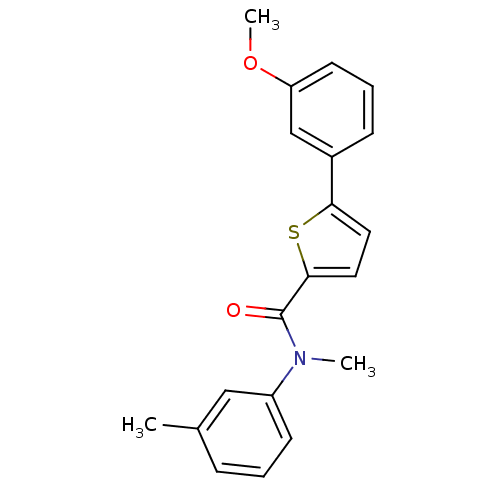 (CHEMBL2324693) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426592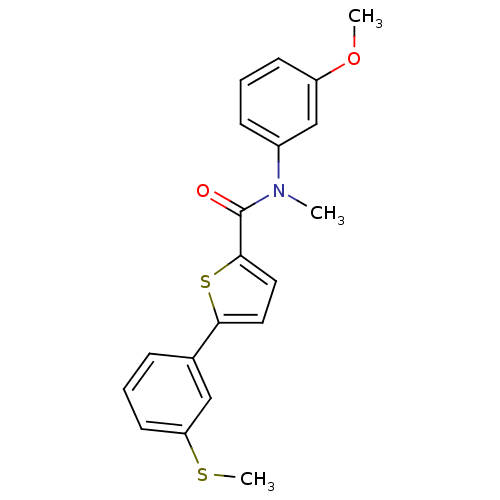 (CHEMBL2324692) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 242 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426584 (CHEMBL2324367) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 242 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426583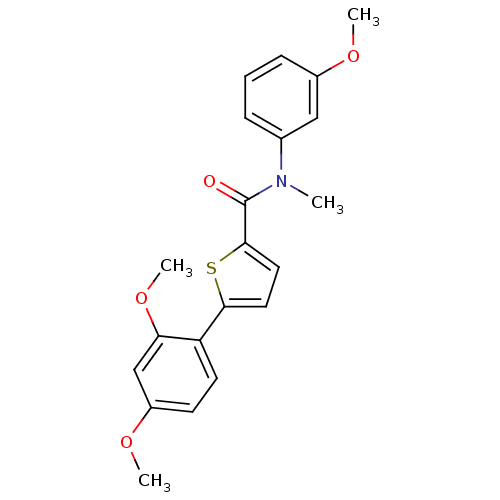 (CHEMBL2324370) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 313 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358108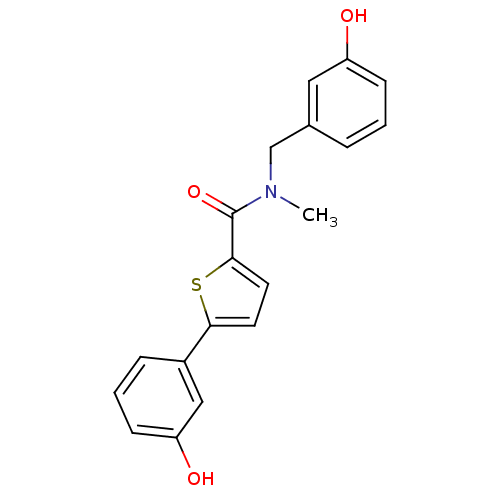 (CHEMBL1915944) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 394 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358111 (CHEMBL1915940) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 482 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426590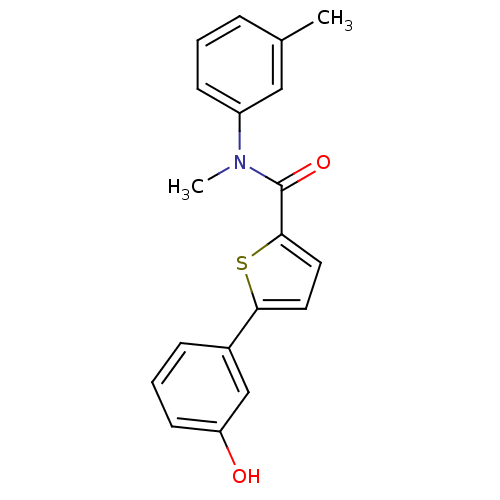 (CHEMBL2324371) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 645 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426589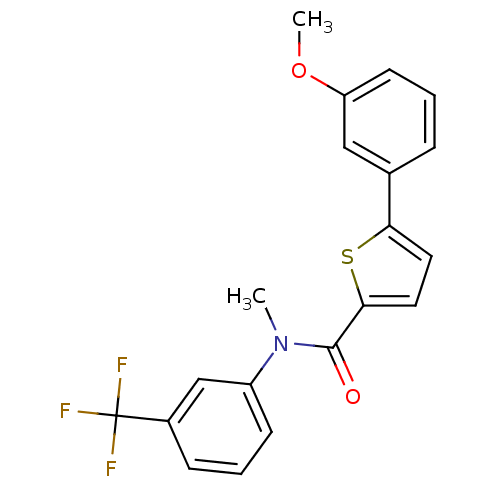 (CHEMBL2324373) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 721 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50358118 (CHEMBL1915964) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in HEK293 cell using [1,2-3H]Cortisone as substrate after 10 mins by scintillation counting ana... | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50426588 (CHEMBL2324673) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD1 in human placental cytosolic fraction using [3H]E1 as substrate assessed as formation of E2 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426595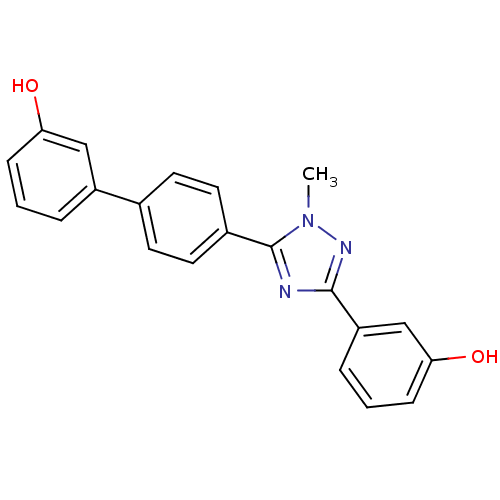 (CHEMBL1915966) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 (unknown origin) | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50426583 (CHEMBL2324370) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD1 in human placental cytosolic fraction using [3H]E1 as substrate assessed as formation of E2 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358111 (CHEMBL1915940) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD1 in human placental cytosolic fraction using [3H]E1 as substrate assessed as formation of E2 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50426594 (CHEMBL2324683) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD1 in human placental cytosolic fraction using [3H]E1 as substrate assessed as formation of E2 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358118 (CHEMBL1915964) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD1 in human placental cytosolic fraction using [3H]E1 as substrate assessed as formation of E2 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50426585 (CHEMBL2324366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD1 in human placental cytosolic fraction using [3H]E1 as substrate assessed as formation of E2 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50426592 (CHEMBL2324692) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD1 in human placental cytosolic fraction using [3H]E1 as substrate assessed as formation of E2 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50426586 (CHEMBL2324365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD1 in human placental cytosolic fraction using [3H]E1 as substrate assessed as formation of E2 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358108 (CHEMBL1915944) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD1 in human placental cytosolic fraction using [3H]E1 as substrate assessed as formation of E2 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50426582 (CHEMBL2324678) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD1 in human placental cytosolic fraction using [3H]E1 as substrate assessed as formation of E2 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50426580 (CHEMBL2324690) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD1 in human placental cytosolic fraction using [3H]E1 as substrate assessed as formation of E2 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50426590 (CHEMBL2324371) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD1 in human placental cytosolic fraction using [3H]E1 as substrate assessed as formation of E2 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50426581 (CHEMBL2324679) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD1 in human placental cytosolic fraction using [3H]E1 as substrate assessed as formation of E2 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50426587 (CHEMBL2324361) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD1 in human placental cytosolic fraction using [3H]E1 as substrate assessed as formation of E2 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50426593 (CHEMBL2324691) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD1 in human placental cytosolic fraction using [3H]E1 as substrate assessed as formation of E2 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50426591 (CHEMBL2324693) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD1 in human placental cytosolic fraction using [3H]E1 as substrate assessed as formation of E2 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50426589 (CHEMBL2324373) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD1 in human placental cytosolic fraction using [3H]E1 as substrate assessed as formation of E2 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50426584 (CHEMBL2324367) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD1 in human placental cytosolic fraction using [3H]E1 as substrate assessed as formation of E2 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50426579 (CHEMBL2324360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD1 in human placental cytosolic fraction using [3H]E1 as substrate assessed as formation of E2 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||