

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50051747 (5-Amino-2-{4-[(2-amino-4-hydroxy-5,6,7,8-tetrahydr...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against mammalian Folyl-polyglutamate synthase | J Med Chem 39: 2536-40 (1996) Article DOI: 10.1021/jm960046w BindingDB Entry DOI: 10.7270/Q2000158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory concentration against dihydrofolate reductase (DHFR) enzyme isolated from CCRF-CEM human leukemia cells. | J Med Chem 39: 2536-40 (1996) Article DOI: 10.1021/jm960046w BindingDB Entry DOI: 10.7270/Q2000158 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50011886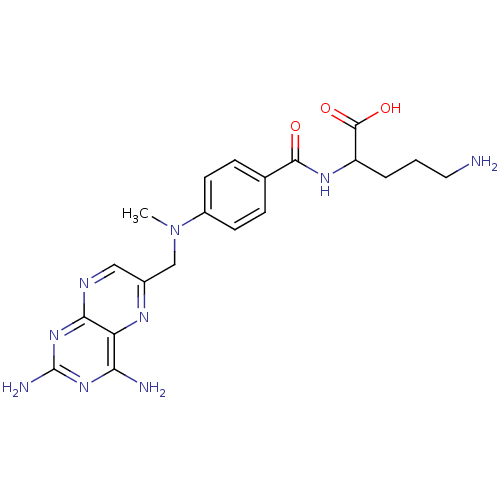 (5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory concentration against dihydrofolate reductase enzyme (DHFR) enzyme isolated from CCRF-CEM human leukemia cells. value mentioned is from li... | J Med Chem 39: 2536-40 (1996) Article DOI: 10.1021/jm960046w BindingDB Entry DOI: 10.7270/Q2000158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50051746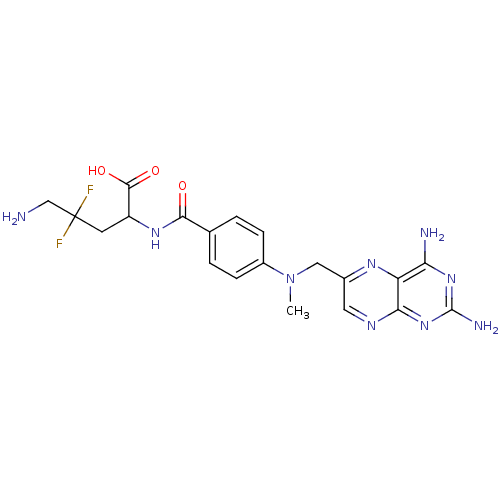 (5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory concentration against dihydrofolate reductase enzyme (DHFR) enzyme isolated from CCRF-CEM human leukemia cells. | J Med Chem 39: 2536-40 (1996) Article DOI: 10.1021/jm960046w BindingDB Entry DOI: 10.7270/Q2000158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50011886 (5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory concentration against dihydrofolate reductase enzyme (DHFR) enzyme isolated from CCRF-CEM human leukemia cells. | J Med Chem 39: 2536-40 (1996) Article DOI: 10.1021/jm960046w BindingDB Entry DOI: 10.7270/Q2000158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50011886 (5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-me...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory concentration against human Folyl-polyglutamate synthase isolated from CCRF-CEM human leukemia cells. | J Med Chem 39: 2536-40 (1996) Article DOI: 10.1021/jm960046w BindingDB Entry DOI: 10.7270/Q2000158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50051746 (5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-me...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory concentration against human Folyl-polyglutamate synthase isolated from CCRF-CEM human leukemia cells. | J Med Chem 39: 2536-40 (1996) Article DOI: 10.1021/jm960046w BindingDB Entry DOI: 10.7270/Q2000158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||