

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor A (RAT) | BDBM50082568 (2-({3-[4-(2-Azido-benzenesulfonylaminocarbonyl)-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Fournier S.A. Curated by ChEMBL | Assay Description The compound was evaluated for the inhibition of [125I]-Sar-AII binding to Angiotensin II receptor, type 1 from purified rat liver membranes. | J Med Chem 42: 4572-83 (1999) BindingDB Entry DOI: 10.7270/Q2XW4J1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50082567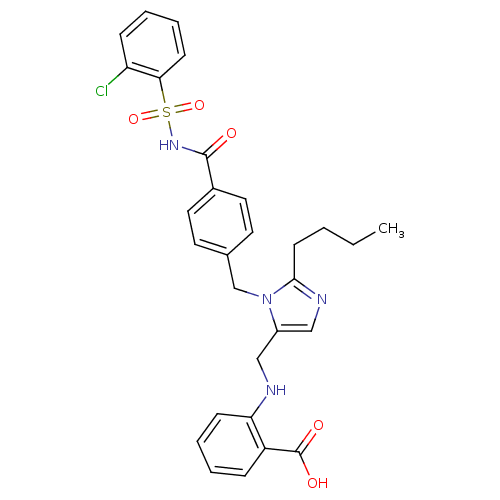 (2-({2-Butyl-3-[4-(2-chloro-benzenesulfonylaminocar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Fournier S.A. Curated by ChEMBL | Assay Description The compound was evaluated for the inhibition of [125I]-Sar-AII binding to Angiotensin II receptor, type 1 from purified rat liver membranes. | J Med Chem 42: 4572-83 (1999) BindingDB Entry DOI: 10.7270/Q2XW4J1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50082570 (2-[2-butyl-1-(4-carboxybenzyl)-1H-5-imidazolylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Fournier S.A. Curated by ChEMBL | Assay Description The compound was evaluated for the inhibition of [125I]-Sar-AII binding to Angiotensin II receptor, type 1 from purified rat liver membranes. | J Med Chem 42: 4572-83 (1999) BindingDB Entry DOI: 10.7270/Q2XW4J1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50082569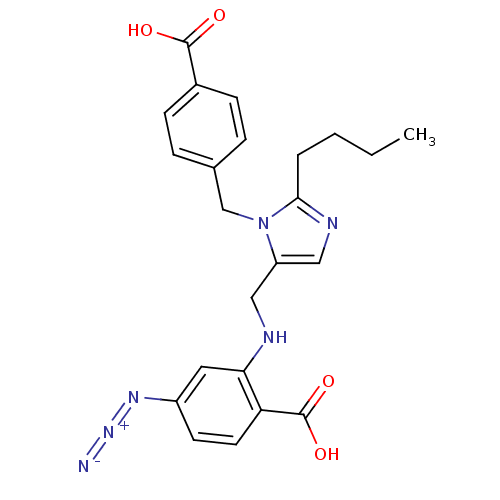 (4-Azido-2-{[2-butyl-3-(4-carboxy-benzyl)-3H-imidaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Fournier S.A. Curated by ChEMBL | Assay Description The compound was evaluated for the inhibition of [125I]-Sar-AII binding to Angiotensin II receptor, type 1 from purified rat liver membranes. | J Med Chem 42: 4572-83 (1999) BindingDB Entry DOI: 10.7270/Q2XW4J1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||