 Found 390 hits Enz. Inhib. hit(s) with all data for entry = 50009520
Found 390 hits Enz. Inhib. hit(s) with all data for entry = 50009520 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084563
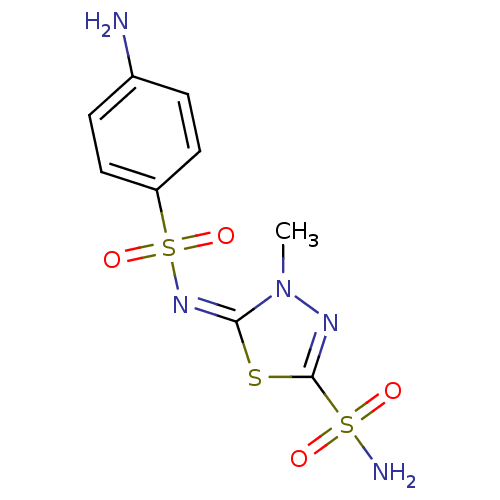
(5-(4-Amino-benzenesulfonylimino)-4-methyl-4,5-dihy...)Show SMILES Cn1nc(s\c1=N\S(=O)(=O)c1ccc(N)cc1)S(N)(=O)=O Show InChI InChI=1S/C9H11N5O4S3/c1-14-8(19-9(12-14)20(11,15)16)13-21(17,18)7-4-2-6(10)3-5-7/h2-5H,10H2,1H3,(H2,11,15,16)/b13-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50084563

(5-(4-Amino-benzenesulfonylimino)-4-methyl-4,5-dihy...)Show SMILES Cn1nc(s\c1=N\S(=O)(=O)c1ccc(N)cc1)S(N)(=O)=O Show InChI InChI=1S/C9H11N5O4S3/c1-14-8(19-9(12-14)20(11,15)16)13-21(17,18)7-4-2-6(10)3-5-7/h2-5H,10H2,1H3,(H2,11,15,16)/b13-8+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV (CA4), isolated from bovine lung microsomes |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50084563

(5-(4-Amino-benzenesulfonylimino)-4-methyl-4,5-dihy...)Show SMILES Cn1nc(s\c1=N\S(=O)(=O)c1ccc(N)cc1)S(N)(=O)=O Show InChI InChI=1S/C9H11N5O4S3/c1-14-8(19-9(12-14)20(11,15)16)13-21(17,18)7-4-2-6(10)3-5-7/h2-5H,10H2,1H3,(H2,11,15,16)/b13-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase I (CA1) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10870

(2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...)Show InChI InChI=1S/C8H9N5O4S3/c9-5-1-3-6(4-2-5)20(16,17)13-7-11-12-8(18-7)19(10,14)15/h1-4H,9H2,(H,11,13)(H2,10,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084561

(2,6-Dimethyl-1-{[4-(3-methyl-5-sulfamoyl-3H-[1,3,4...)Show SMILES Cc1cc(cc(C)[n+]1CC(=O)Nc1ccc(cc1)S(=O)(=O)\N=c1\sc(nn1C)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C24H24N6O5S3/c1-16-13-19(18-7-5-4-6-8-18)14-17(2)30(16)15-22(31)26-20-9-11-21(12-10-20)38(34,35)28-23-29(3)27-24(36-23)37(25,32)33/h4-14H,15H2,1-3H3,(H2-,25,26,31,32,33)/p+1/b28-23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084560

(2,6-Dimethyl-4-phenyl-1-{[4-(5-sulfamoyl-[1,3,4]th...)Show SMILES Cc1cc(cc(C)[n+]1CC(=O)Nc1ccc(cc1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C23H22N6O5S3/c1-15-12-18(17-6-4-3-5-7-17)13-16(2)29(15)14-21(30)25-19-8-10-20(11-9-19)37(33,34)28-22-26-27-23(35-22)36(24,31)32/h3-13H,14H2,1-2H3,(H3-,24,25,26,28,30,31,32)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50079068
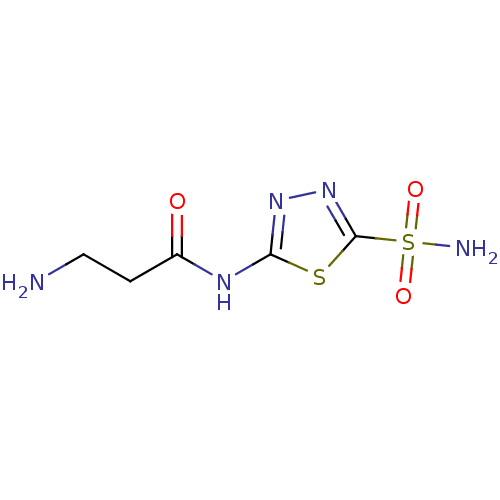
(3-amino-N-(5-sulfamoyl-[1,3,4]thiadiazol-2-yl)-pro...)Show InChI InChI=1S/C5H9N5O3S2/c6-2-1-3(11)8-4-9-10-5(14-4)15(7,12)13/h1-2,6H2,(H2,7,12,13)(H,8,9,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084509
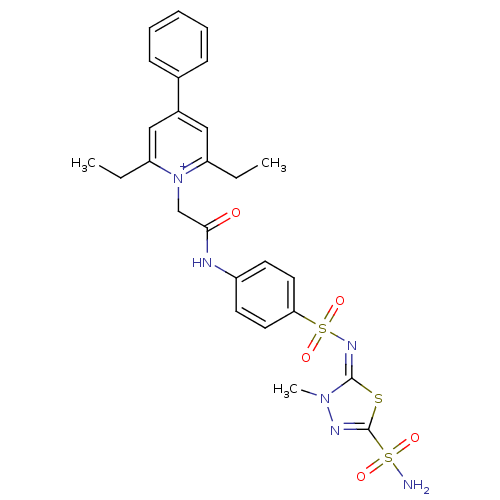
(2,6-Diethyl-1-{[4-(3-methyl-5-sulfamoyl-3H-[1,3,4]...)Show SMILES CCc1cc(cc(CC)[n+]1CC(=O)Nc1ccc(cc1)S(=O)(=O)\N=c1\sc(nn1C)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C26H28N6O5S3/c1-4-21-15-19(18-9-7-6-8-10-18)16-22(5-2)32(21)17-24(33)28-20-11-13-23(14-12-20)40(36,37)30-25-31(3)29-26(38-25)39(27,34)35/h6-16H,4-5,17H2,1-3H3,(H2-,27,28,33,34,35)/p+1/b30-25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084512
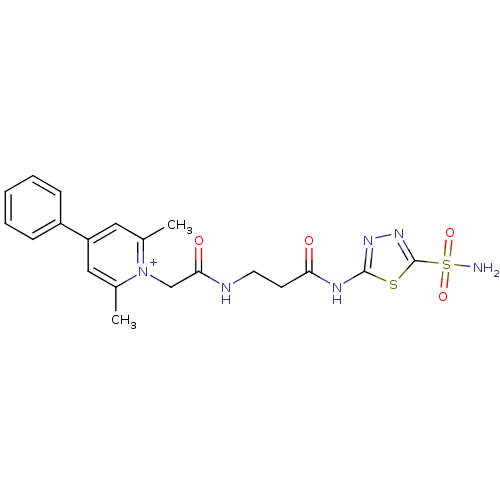
(2,6-Dimethyl-4-phenyl-1-{[2-(5-sulfamoyl-[1,3,4]th...)Show SMILES Cc1cc(cc(C)[n+]1CC(=O)NCCC(=O)Nc1nnc(s1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C20H22N6O4S2/c1-13-10-16(15-6-4-3-5-7-15)11-14(2)26(13)12-18(28)22-9-8-17(27)23-19-24-25-20(31-19)32(21,29)30/h3-7,10-11H,8-9,12H2,1-2H3,(H3-,21,22,23,24,27,28,29,30)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084554

(2,6-Diisopropyl-1-{[4-(3-methyl-5-sulfamoyl-3H-[1,...)Show SMILES CC(C)c1cc(cc(C(C)C)[n+]1CC(=O)Nc1ccc(cc1)S(=O)(=O)\N=c1\sc(nn1C)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C28H32N6O5S3/c1-18(2)24-15-21(20-9-7-6-8-10-20)16-25(19(3)4)34(24)17-26(35)30-22-11-13-23(14-12-22)42(38,39)32-27-33(5)31-28(40-27)41(29,36)37/h6-16,18-19H,17H2,1-5H3,(H2-,29,30,35,36,37)/p+1/b32-27+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084534
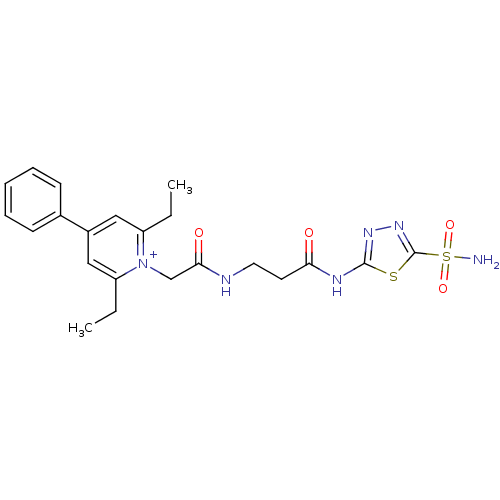
(2,6-Diethyl-4-phenyl-1-{[2-(5-sulfamoyl-[1,3,4]thi...)Show SMILES CCc1cc(cc(CC)[n+]1CC(=O)NCCC(=O)Nc1nnc(s1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C22H26N6O4S2/c1-3-17-12-16(15-8-6-5-7-9-15)13-18(4-2)28(17)14-20(30)24-11-10-19(29)25-21-26-27-22(33-21)34(23,31)32/h5-9,12-13H,3-4,10-11,14H2,1-2H3,(H3-,23,24,25,26,29,30,31,32)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084576
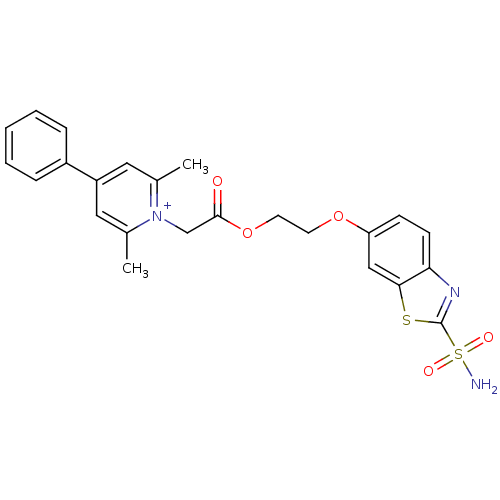
(2,6-Dimethyl-4-phenyl-1-[2-(2-sulfamoyl-benzothiaz...)Show SMILES Cc1cc(cc(C)[n+]1CC(=O)OCCOc1ccc2nc(sc2c1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C24H24N3O5S2/c1-16-12-19(18-6-4-3-5-7-18)13-17(2)27(16)15-23(28)32-11-10-31-20-8-9-21-22(14-20)33-24(26-21)34(25,29)30/h3-9,12-14H,10-11,15H2,1-2H3,(H2,25,29,30)/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084525
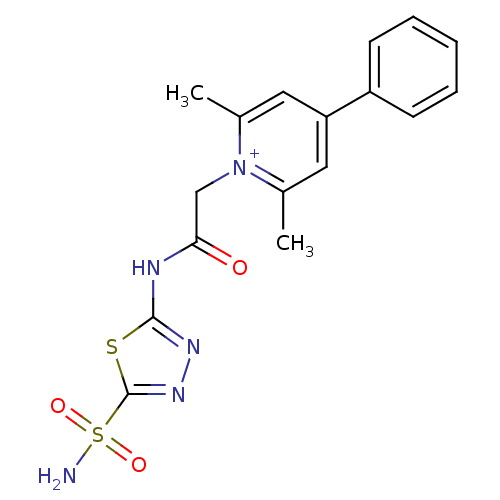
(2,6-Dimethyl-4-phenyl-1-[(5-sulfamoyl-[1,3,4]thiad...)Show SMILES Cc1cc(cc(C)[n+]1CC(=O)Nc1nnc(s1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C17H17N5O3S2/c1-11-8-14(13-6-4-3-5-7-13)9-12(2)22(11)10-15(23)19-16-20-21-17(26-16)27(18,24)25/h3-9H,10H2,1-2H3,(H2-,18,19,20,23,24,25)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084514

(2,6-Diisopropyl-4-phenyl-1-{[4-(5-sulfamoyl-[1,3,4...)Show SMILES CC(C)c1cc(cc(C(C)C)[n+]1CC(=O)Nc1ccc(cc1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C27H30N6O5S3/c1-17(2)23-14-20(19-8-6-5-7-9-19)15-24(18(3)4)33(23)16-25(34)29-21-10-12-22(13-11-21)41(37,38)32-26-30-31-27(39-26)40(28,35)36/h5-15,17-18H,16H2,1-4H3,(H3-,28,29,30,32,34,35,36)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084552

(2,6-Diethyl-4-phenyl-1-[2-(2-sulfamoyl-benzothiazo...)Show SMILES CCc1cc(cc(CC)[n+]1CC(=O)OCCOc1ccc2nc(sc2c1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C26H28N3O5S2/c1-3-20-14-19(18-8-6-5-7-9-18)15-21(4-2)29(20)17-25(30)34-13-12-33-22-10-11-23-24(16-22)35-26(28-23)36(27,31)32/h5-11,14-16H,3-4,12-13,17H2,1-2H3,(H2,27,31,32)/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084543

(2,4,6-Trimethyl-1-{[4-(3-methyl-5-sulfamoyl-3H-[1,...)Show SMILES Cc1cc(C)[n+](CC(=O)Nc2ccc(cc2)S(=O)(=O)\N=c2\sc(nn2C)S(N)(=O)=O)c(C)c1 Show InChI InChI=1S/C19H22N6O5S3/c1-12-9-13(2)25(14(3)10-12)11-17(26)21-15-5-7-16(8-6-15)33(29,30)23-18-24(4)22-19(31-18)32(20,27)28/h5-10H,11H2,1-4H3,(H2-,20,21,26,27,28)/p+1/b23-18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084540

(2,6-Dimethyl-4-phenyl-1-[(2-sulfamoyl-benzothiazol...)Show SMILES Cc1cc(cc(C)[n+]1CC(=O)Nc1ccc2nc(sc2c1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C22H20N4O3S2/c1-14-10-17(16-6-4-3-5-7-16)11-15(2)26(14)13-21(27)24-18-8-9-19-20(12-18)30-22(25-19)31(23,28)29/h3-12H,13H2,1-2H3,(H2-,23,24,27,28,29)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084608
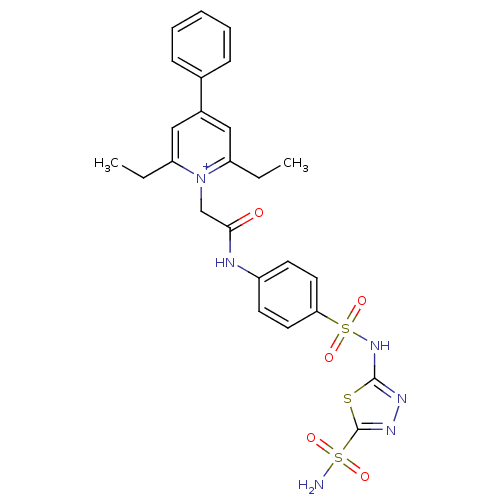
(2,6-Diethyl-4-phenyl-1-{[4-(5-sulfamoyl-[1,3,4]thi...)Show SMILES CCc1cc(cc(CC)[n+]1CC(=O)Nc1ccc(cc1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C25H26N6O5S3/c1-3-20-14-18(17-8-6-5-7-9-17)15-21(4-2)31(20)16-23(32)27-19-10-12-22(13-11-19)39(35,36)30-24-28-29-25(37-24)38(26,33)34/h5-15H,3-4,16H2,1-2H3,(H3-,26,27,28,30,32,33,34)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084592

(2,6-Dimethyl-4-phenyl-1-(2-sulfamoyl-benzothiazol-...)Show SMILES Cc1cc(cc(C)[n+]1CC(=O)Oc1ccc2nc(sc2c1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C22H20N3O4S2/c1-14-10-17(16-6-4-3-5-7-16)11-15(2)25(14)13-21(26)29-18-8-9-19-20(12-18)30-22(24-19)31(23,27)28/h3-12H,13H2,1-2H3,(H2,23,27,28)/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM10870

(2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...)Show InChI InChI=1S/C8H9N5O4S3/c9-5-1-3-6(4-2-5)20(16,17)13-7-11-12-8(18-7)19(10,14)15/h1-4H,9H2,(H,11,13)(H2,10,14,15) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV (CA4), isolated from bovine lung microsomes |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084537

(2,6-Dimethyl-1-[(3-methyl-5-sulfamoyl-3H-[1,3,4]th...)Show SMILES Cc1cc(cc(C)[n+]1CC(=O)N=c1sc(nn1C)S(N)(=O)=O)-c1ccccc1 |w:11.11| Show InChI InChI=1S/C18H20N5O3S2/c1-12-9-15(14-7-5-4-6-8-14)10-13(2)23(12)11-16(24)20-17-22(3)21-18(27-17)28(19,25)26/h4-10H,11H2,1-3H3,(H2,19,25,26)/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084517
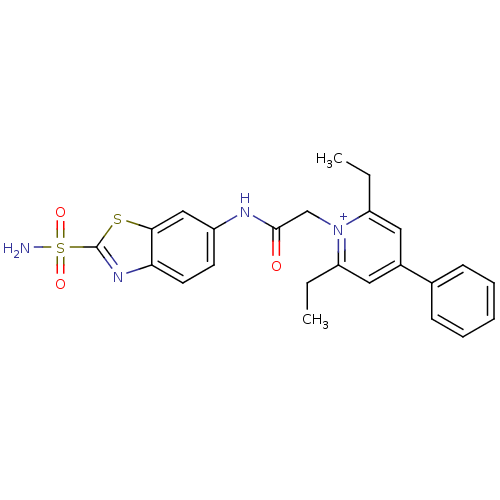
(2,6-Diethyl-4-phenyl-1-[(2-sulfamoyl-benzothiazol-...)Show SMILES CCc1cc(cc(CC)[n+]1CC(=O)Nc1ccc2nc(sc2c1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C24H24N4O3S2/c1-3-19-12-17(16-8-6-5-7-9-16)13-20(4-2)28(19)15-23(29)26-18-10-11-21-22(14-18)32-24(27-21)33(25,30)31/h5-14H,3-4,15H2,1-2H3,(H2-,25,26,29,30,31)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10870

(2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...)Show InChI InChI=1S/C8H9N5O4S3/c9-5-1-3-6(4-2-5)20(16,17)13-7-11-12-8(18-7)19(10,14)15/h1-4H,9H2,(H,11,13)(H2,10,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase I (CA1) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080733
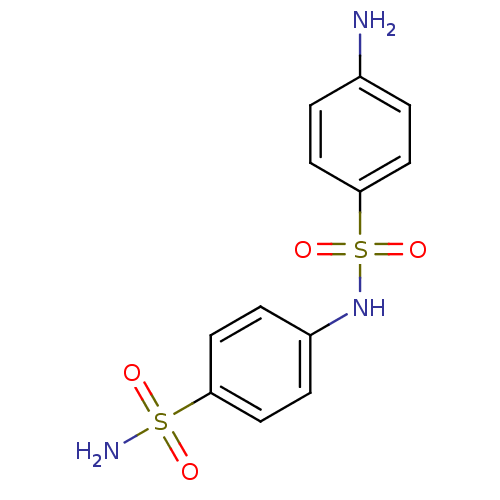
(4-(4-aminophenylsulfonamido)-1-benzenesulfonamide ...)Show InChI InChI=1S/C12H13N3O4S2/c13-9-1-5-12(6-2-9)21(18,19)15-10-3-7-11(8-4-10)20(14,16)17/h1-8,15H,13H2,(H2,14,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084569

(2,6-Diisopropyl-4-phenyl-1-{[2-(5-sulfamoyl-[1,3,4...)Show SMILES CC(C)c1cc(cc(C(C)C)[n+]1CC(=O)NCCC(=O)Nc1nnc(s1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C24H30N6O4S2/c1-15(2)19-12-18(17-8-6-5-7-9-17)13-20(16(3)4)30(19)14-22(32)26-11-10-21(31)27-23-28-29-24(35-23)36(25,33)34/h5-9,12-13,15-16H,10-11,14H2,1-4H3,(H3-,25,26,27,28,31,32,33,34)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084510
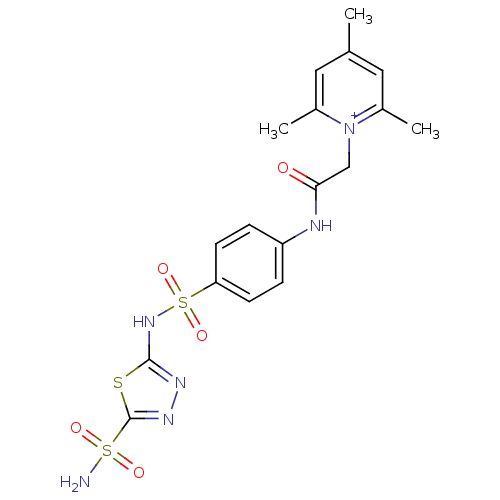
(2,4,6-Trimethyl-1-{[4-(5-sulfamoyl-[1,3,4]thiadiaz...)Show SMILES Cc1cc(C)[n+](CC(=O)Nc2ccc(cc2)S(=O)(=O)Nc2nnc(s2)S(N)(=O)=O)c(C)c1 Show InChI InChI=1S/C18H20N6O5S3/c1-11-8-12(2)24(13(3)9-11)10-16(25)20-14-4-6-15(7-5-14)32(28,29)23-17-21-22-18(30-17)31(19,26)27/h4-9H,10H2,1-3H3,(H3-,19,20,21,23,25,26,27)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084533
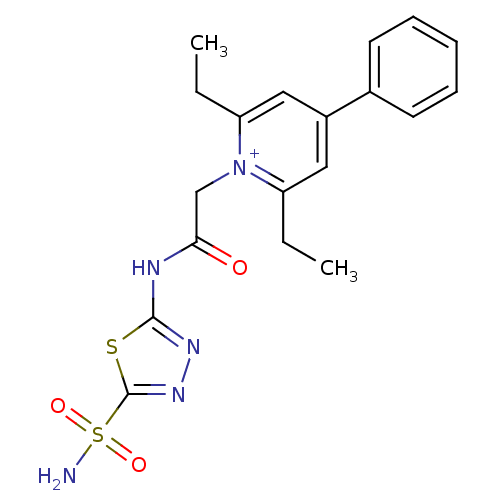
(2,6-Diethyl-4-phenyl-1-[(5-sulfamoyl-[1,3,4]thiadi...)Show SMILES CCc1cc(cc(CC)[n+]1CC(=O)Nc1nnc(s1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C19H21N5O3S2/c1-3-15-10-14(13-8-6-5-7-9-13)11-16(4-2)24(15)12-17(25)21-18-22-23-19(28-18)29(20,26)27/h5-11H,3-4,12H2,1-2H3,(H2-,20,21,22,25,26,27)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084571
(2,4,6-Trimethyl-1-[2-(2-sulfamoyl-benzothiazol-6-y...)Show SMILES Cc1cc(C)[n+](CC(=O)OCCOc2ccc3nc(sc3c2)S(N)(=O)=O)c(C)c1 Show InChI InChI=1S/C19H22N3O5S2/c1-12-8-13(2)22(14(3)9-12)11-18(23)27-7-6-26-15-4-5-16-17(10-15)28-19(21-16)29(20,24)25/h4-5,8-10H,6-7,11H2,1-3H3,(H2,20,24,25)/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084562
(2,6-Diisopropyl-4-phenyl-1-[2-(2-sulfamoyl-benzoth...)Show SMILES CC(C)c1cc(cc(C(C)C)[n+]1CC(=O)OCCOc1ccc2nc(sc2c1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C28H32N3O5S2/c1-18(2)24-14-21(20-8-6-5-7-9-20)15-25(19(3)4)31(24)17-27(32)36-13-12-35-22-10-11-23-26(16-22)37-28(30-23)38(29,33)34/h5-11,14-16,18-19H,12-13,17H2,1-4H3,(H2,29,33,34)/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084605
(2,4,6-Trimethyl-1-{[2-(5-sulfamoyl-[1,3,4]thiadiaz...)Show SMILES Cc1cc(C)[n+](CC(=O)NCCC(=O)Nc2nnc(s2)S(N)(=O)=O)c(C)c1 Show InChI InChI=1S/C15H20N6O4S2/c1-9-6-10(2)21(11(3)7-9)8-13(23)17-5-4-12(22)18-14-19-20-15(26-14)27(16,24)25/h6-7H,4-5,8H2,1-3H3,(H3-,16,17,18,19,22,23,24,25)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50079049
(6-(2-Hydroxy-ethoxy)-benzothiazole-2-sulfonic acid...)Show InChI InChI=1S/C9H10N2O4S2/c10-17(13,14)9-11-7-2-1-6(15-4-3-12)5-8(7)16-9/h1-2,5,12H,3-4H2,(H2,10,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084523

(2,6-Diethyl-4-phenyl-1-(2-sulfamoyl-benzothiazol-6...)Show SMILES CCc1cc(cc(CC)[n+]1CC(=O)Oc1ccc2nc(sc2c1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C24H24N3O4S2/c1-3-18-12-17(16-8-6-5-7-9-16)13-19(4-2)27(18)15-23(28)31-20-10-11-21-22(14-20)32-24(26-21)33(25,29)30/h5-14H,3-4,15H2,1-2H3,(H2,25,29,30)/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084607

(2,6-Diethyl-1-[(3-methyl-5-sulfamoyl-3H-[1,3,4]thi...)Show SMILES CCc1cc(cc(CC)[n+]1CC(=O)N=c1sc(nn1C)S(N)(=O)=O)-c1ccccc1 |w:13.13| Show InChI InChI=1S/C20H24N5O3S2/c1-4-16-11-15(14-9-7-6-8-10-14)12-17(5-2)25(16)13-18(26)22-19-24(3)23-20(29-19)30(21,27)28/h6-12H,4-5,13H2,1-3H3,(H2,21,27,28)/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084595
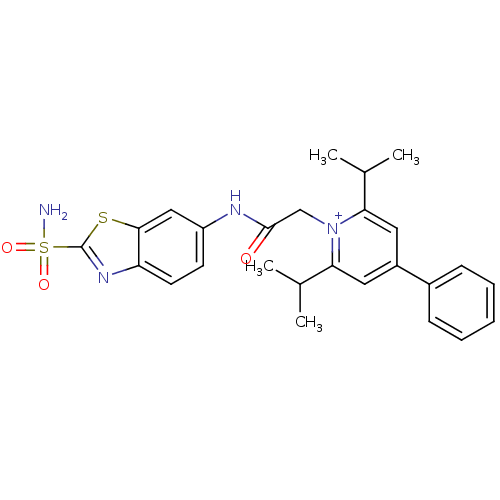
(2,6-Diisopropyl-4-phenyl-1-[(2-sulfamoyl-benzothia...)Show SMILES CC(C)c1cc(cc(C(C)C)[n+]1CC(=O)Nc1ccc2nc(sc2c1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C26H28N4O3S2/c1-16(2)22-12-19(18-8-6-5-7-9-18)13-23(17(3)4)30(22)15-25(31)28-20-10-11-21-24(14-20)34-26(29-21)35(27,32)33/h5-14,16-17H,15H2,1-4H3,(H2-,27,28,31,32,33)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084587
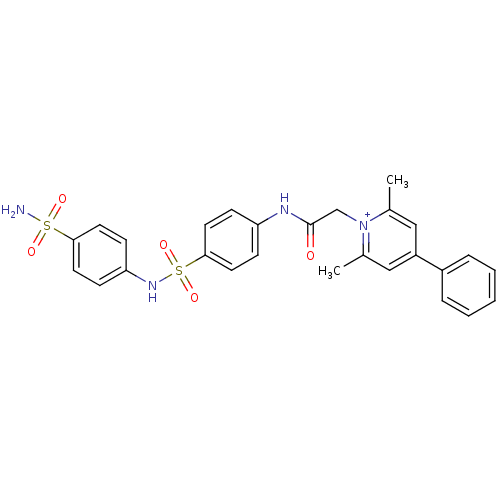
(2,6-Dimethyl-4-phenyl-1-{[4-(4-sulfamoyl-phenylsul...)Show SMILES Cc1cc(cc(C)[n+]1CC(=O)Nc1ccc(cc1)S(=O)(=O)Nc1ccc(cc1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C27H26N4O5S2/c1-19-16-22(21-6-4-3-5-7-21)17-20(2)31(19)18-27(32)29-23-8-14-26(15-9-23)38(35,36)30-24-10-12-25(13-11-24)37(28,33)34/h3-17,30H,18H2,1-2H3,(H2-,28,29,32,33,34)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
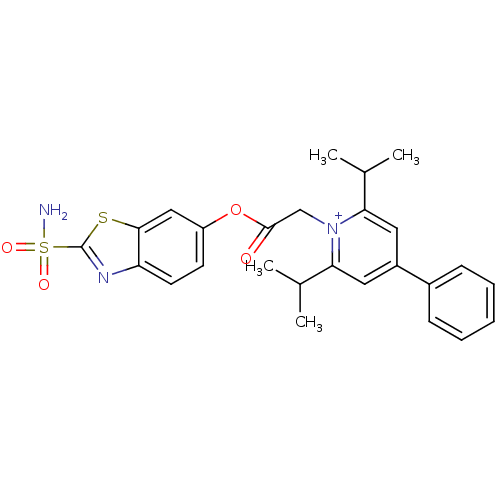
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084539
(2,6-Diisopropyl-4-phenyl-1-(2-sulfamoyl-benzothiaz...)Show SMILES CC(C)c1cc(cc(C(C)C)[n+]1CC(=O)Oc1ccc2nc(sc2c1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C26H28N3O4S2/c1-16(2)22-12-19(18-8-6-5-7-9-18)13-23(17(3)4)29(22)15-25(30)33-20-10-11-21-24(14-20)34-26(28-21)35(27,31)32/h5-14,16-17H,15H2,1-4H3,(H2,27,31,32)/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50084561

(2,6-Dimethyl-1-{[4-(3-methyl-5-sulfamoyl-3H-[1,3,4...)Show SMILES Cc1cc(cc(C)[n+]1CC(=O)Nc1ccc(cc1)S(=O)(=O)\N=c1\sc(nn1C)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C24H24N6O5S3/c1-16-13-19(18-7-5-4-6-8-18)14-17(2)30(16)15-22(31)26-20-9-11-21(12-10-20)38(34,35)28-23-29(3)27-24(36-23)37(25,32)33/h4-14H,15H2,1-3H3,(H2-,25,26,31,32,33)/p+1/b28-23+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV (CA4), isolated from bovine lung microsomes |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084556
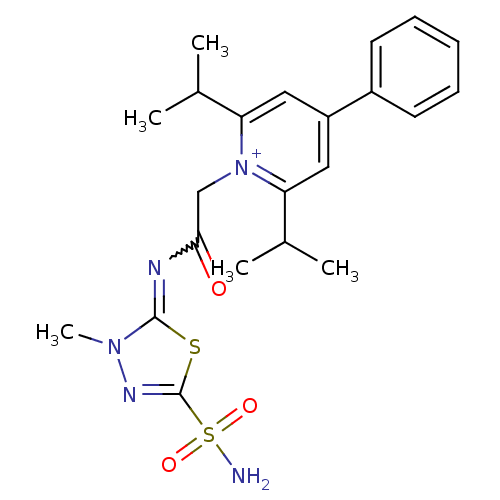
(2,6-Diisopropyl-1-[(3-methyl-5-sulfamoyl-3H-[1,3,4...)Show SMILES CC(C)c1cc(cc(C(C)C)[n+]1CC(=O)N=c1sc(nn1C)S(N)(=O)=O)-c1ccccc1 |w:15.15| Show InChI InChI=1S/C22H28N5O3S2/c1-14(2)18-11-17(16-9-7-6-8-10-16)12-19(15(3)4)27(18)13-20(28)24-21-26(5)25-22(31-21)32(23,29)30/h6-12,14-15H,13H2,1-5H3,(H2,23,29,30)/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10874
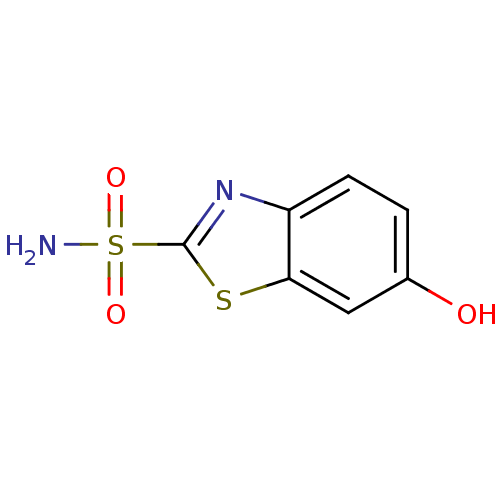
(6-hydroxy-1,3-benzothiazole-2-sulfonamide | CHEMBL...)Show InChI InChI=1S/C7H6N2O3S2/c8-14(11,12)7-9-5-2-1-4(10)3-6(5)13-7/h1-3,10H,(H2,8,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084555
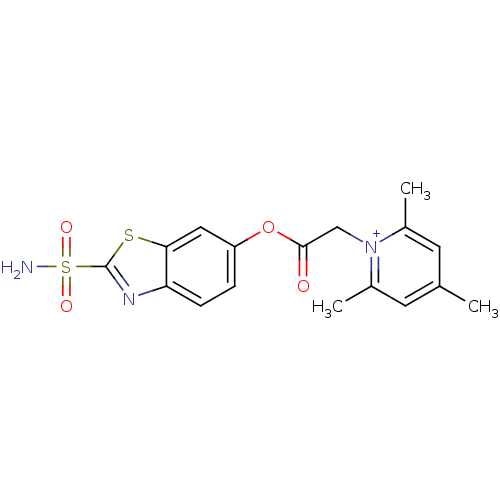
(2,4,6-Trimethyl-1-(2-sulfamoyl-benzothiazol-6-ylox...)Show SMILES Cc1cc(C)[n+](CC(=O)Oc2ccc3nc(sc3c2)S(N)(=O)=O)c(C)c1 Show InChI InChI=1S/C17H18N3O4S2/c1-10-6-11(2)20(12(3)7-10)9-16(21)24-13-4-5-14-15(8-13)25-17(19-14)26(18,22)23/h4-8H,9H2,1-3H3,(H2,18,22,23)/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084511
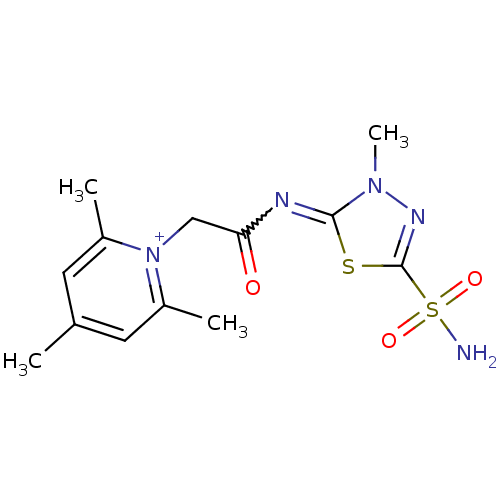
(2,4,6-Trimethyl-1-[(3-methyl-5-sulfamoyl-3H-[1,3,4...)Show SMILES Cc1cc(C)[n+](CC(=O)N=c2sc(nn2C)S(N)(=O)=O)c(C)c1 |w:9.8| Show InChI InChI=1S/C13H18N5O3S2/c1-8-5-9(2)18(10(3)6-8)7-11(19)15-12-17(4)16-13(22-12)23(14,20)21/h5-6H,7H2,1-4H3,(H2,14,20,21)/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084609
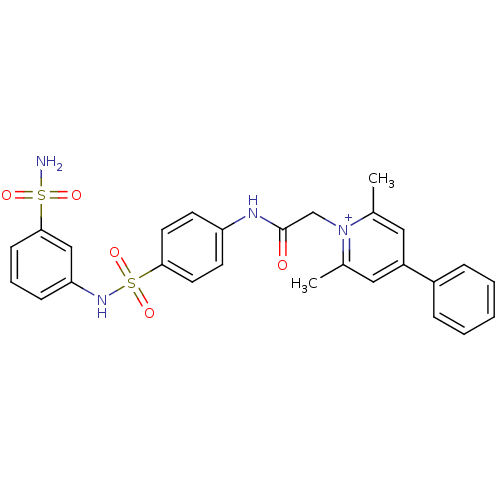
(2,6-Dimethyl-4-phenyl-1-{[4-(3-sulfamoyl-phenylsul...)Show SMILES Cc1cc(cc(C)[n+]1CC(=O)Nc1ccc(cc1)S(=O)(=O)Nc1cccc(c1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C27H26N4O5S2/c1-19-15-22(21-7-4-3-5-8-21)16-20(2)31(19)18-27(32)29-23-11-13-25(14-12-23)38(35,36)30-24-9-6-10-26(17-24)37(28,33)34/h3-17,30H,18H2,1-2H3,(H2-,28,29,32,33,34)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50084608

(2,6-Diethyl-4-phenyl-1-{[4-(5-sulfamoyl-[1,3,4]thi...)Show SMILES CCc1cc(cc(CC)[n+]1CC(=O)Nc1ccc(cc1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C25H26N6O5S3/c1-3-20-14-18(17-8-6-5-7-9-17)15-21(4-2)31(20)16-23(32)27-19-10-12-22(13-11-19)39(35,36)30-24-28-29-25(37-24)38(26,33)34/h5-15H,3-4,16H2,1-2H3,(H3-,26,27,28,30,32,33,34)/p+1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV (CA4), isolated from bovine lung microsomes |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50079035
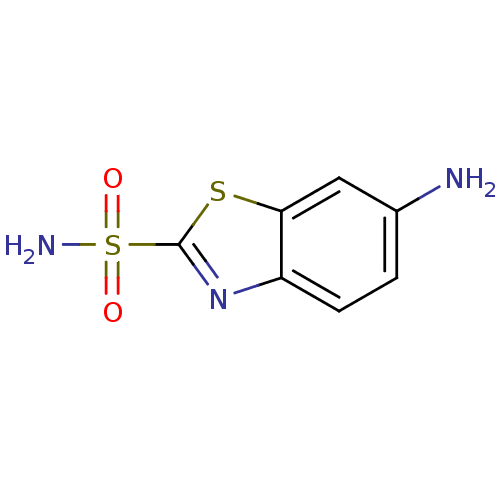
(6-Amino-benzothiazole-2-sulfonic acid amide | 6-am...)Show InChI InChI=1S/C7H7N3O2S2/c8-4-1-2-5-6(3-4)13-7(10-5)14(9,11)12/h1-3H,8H2,(H2,9,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084519

(2,4,6-Trimethyl-1-[(2-sulfamoyl-benzothiazol-6-ylc...)Show SMILES Cc1cc(C)[n+](CC(=O)Nc2ccc3nc(sc3c2)S(N)(=O)=O)c(C)c1 Show InChI InChI=1S/C17H18N4O3S2/c1-10-6-11(2)21(12(3)7-10)9-16(22)19-13-4-5-14-15(8-13)25-17(20-14)26(18,23)24/h4-8H,9H2,1-3H3,(H2-,18,19,22,23,24)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080728
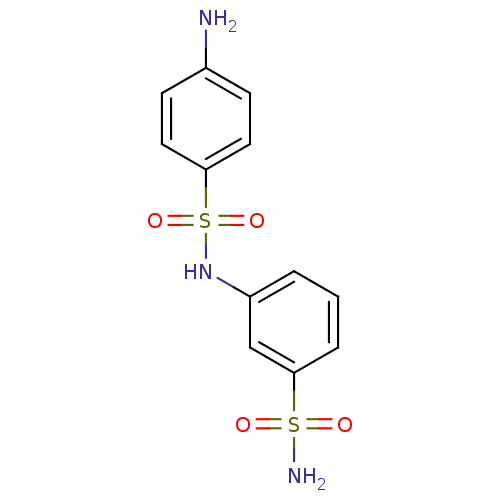
(3-{[(4-aminophenyl)sulfonyl]amino}benzenesulfonami...)Show InChI InChI=1S/C12H13N3O4S2/c13-9-4-6-11(7-5-9)21(18,19)15-10-2-1-3-12(8-10)20(14,16)17/h1-8,15H,13H2,(H2,14,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50084518

(2,6-Diisopropyl-4-phenyl-1-[(5-sulfamoyl-[1,3,4]th...)Show SMILES CC(C)c1cc(cc(C(C)C)[n+]1CC(=O)Nc1nnc(s1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C21H25N5O3S2/c1-13(2)17-10-16(15-8-6-5-7-9-15)11-18(14(3)4)26(17)12-19(27)23-20-24-25-21(30-20)31(22,28)29/h5-11,13-14H,12H2,1-4H3,(H2-,22,23,24,27,28,29)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II (CA2) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50084576

(2,6-Dimethyl-4-phenyl-1-[2-(2-sulfamoyl-benzothiaz...)Show SMILES Cc1cc(cc(C)[n+]1CC(=O)OCCOc1ccc2nc(sc2c1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C24H24N3O5S2/c1-16-12-19(18-6-4-3-5-7-18)13-17(2)27(16)15-23(28)32-11-10-31-20-8-9-21-22(14-20)33-24(26-21)34(25,29)30/h3-9,12-14H,10-11,15H2,1-2H3,(H2,25,29,30)/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase I (CA1) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50084560

(2,6-Dimethyl-4-phenyl-1-{[4-(5-sulfamoyl-[1,3,4]th...)Show SMILES Cc1cc(cc(C)[n+]1CC(=O)Nc1ccc(cc1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C23H22N6O5S3/c1-15-12-18(17-6-4-3-5-7-17)13-16(2)29(15)14-21(30)25-19-8-10-20(11-9-19)37(33,34)28-22-26-27-23(35-22)36(24,31)32/h3-13H,14H2,1-2H3,(H3-,24,25,26,28,30,31,32)/p+1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV (CA4), isolated from bovine lung microsomes |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50084560

(2,6-Dimethyl-4-phenyl-1-{[4-(5-sulfamoyl-[1,3,4]th...)Show SMILES Cc1cc(cc(C)[n+]1CC(=O)Nc1ccc(cc1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C23H22N6O5S3/c1-15-12-18(17-6-4-3-5-7-17)13-16(2)29(15)14-21(30)25-19-8-10-20(11-9-19)37(33,34)28-22-26-27-23(35-22)36(24,31)32/h3-13H,14H2,1-2H3,(H3-,24,25,26,28,30,31,32)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase I (CA1) |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data