 Found 49 hits Enz. Inhib. hit(s) with all data for entry = 50046643
Found 49 hits Enz. Inhib. hit(s) with all data for entry = 50046643 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aromatase
(Homo sapiens (Human)) | BDBM50123025
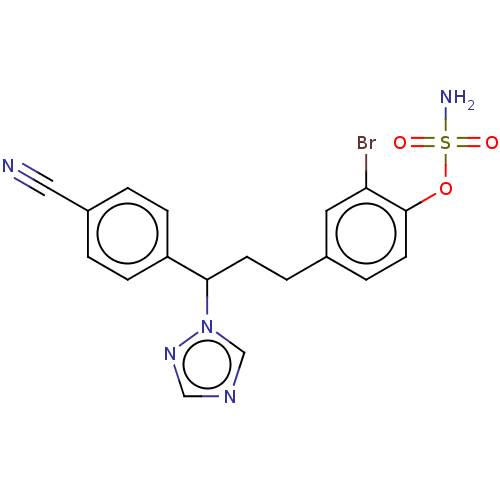
(CHEMBL3623231)Show SMILES NS(=O)(=O)Oc1ccc(CCC(c2ccc(cc2)C#N)n2cncn2)cc1Br Show InChI InChI=1S/C18H16BrN5O3S/c19-16-9-13(4-8-18(16)27-28(21,25)26)3-7-17(24-12-22-11-23-24)15-5-1-14(10-20)2-6-15/h1-2,4-6,8-9,11-12,17H,3,7H2,(H2,21,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human JEG-3 cells using [1beta-3H]androstenedione as substrate after 1 hr by scintillation spectrometry |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123026
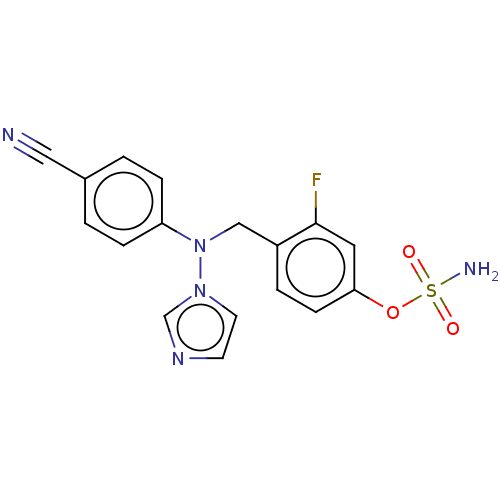
(CHEMBL3623232)Show SMILES NS(=O)(=O)Oc1ccc(CN(c2ccc(cc2)C#N)n2ccnc2)c(F)c1 Show InChI InChI=1S/C17H14FN5O3S/c18-17-9-16(26-27(20,24)25)6-3-14(17)11-23(22-8-7-21-12-22)15-4-1-13(10-19)2-5-15/h1-9,12H,11H2,(H2,20,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human JEG-3 cells using [1beta-3H]androstenedione as substrate after 1 hr by scintillation spectrometry |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50123026

(CHEMBL3623232)Show SMILES NS(=O)(=O)Oc1ccc(CN(c2ccc(cc2)C#N)n2ccnc2)c(F)c1 Show InChI InChI=1S/C17H14FN5O3S/c18-17-9-16(26-27(20,24)25)6-3-14(17)11-23(22-8-7-21-12-22)15-4-1-13(10-19)2-5-15/h1-9,12H,11H2,(H2,20,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human JEG-3 cells using [6,7-3H]E1S as substrate after 1 hr by scintillation spectrometry |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM24306
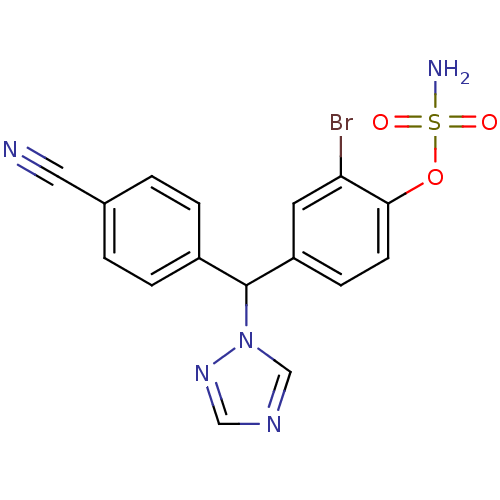
(Letrozole derivative, 40 | {2-bromo-4-[(4-cyanophe...)Show SMILES NS(=O)(=O)Oc1ccc(cc1Br)C(c1ccc(cc1)C#N)n1cncn1 Show InChI InChI=1S/C16H12BrN5O3S/c17-14-7-13(5-6-15(14)25-26(19,23)24)16(22-10-20-9-21-22)12-3-1-11(8-18)2-4-12/h1-7,9-10,16H,(H2,19,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human JEG-3 cells using [1beta-3H]androstenedione as substrate after 1 hr by scintillation spectrometry |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50398452
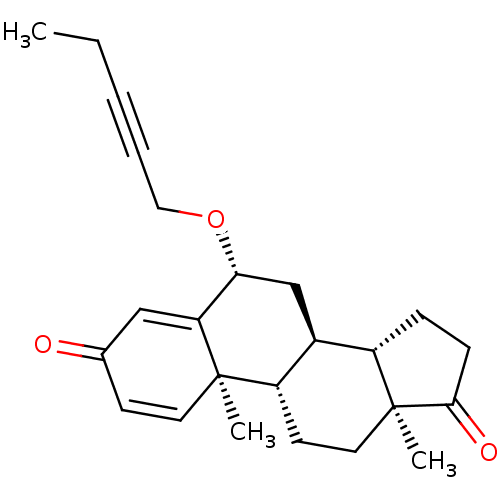
(CHEMBL2179110)Show SMILES CCC#CCO[C@@H]1C[C@H]2[C@@H]3CCC(=O)[C@@]3(C)CC[C@@H]2[C@@]2(C)C=CC(=O)C=C12 |r,c:23,t:27| Show InChI InChI=1S/C24H30O3/c1-4-5-6-13-27-21-15-17-18-7-8-22(26)24(18,3)12-10-19(17)23(2)11-9-16(25)14-20(21)23/h9,11,14,17-19,21H,4,7-8,10,12-13,15H2,1-3H3/t17-,18-,19-,21+,23+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aromatase
(Homo sapiens (Human)) | BDBM50035204

(4-(6-Methoxy-1a,2,3,7b-tetrahydro-1H-cyclopropa[a]...)Show InChI InChI=1S/C17H17NO/c1-19-13-4-2-11-3-5-14-16(17(14)15(11)10-13)12-6-8-18-9-7-12/h2,4,6-10,14,16-17H,3,5H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of human placental aromatase |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50035204

(4-(6-Methoxy-1a,2,3,7b-tetrahydro-1H-cyclopropa[a]...)Show InChI InChI=1S/C17H17NO/c1-19-13-4-2-11-3-5-14-16(17(14)15(11)10-13)12-6-8-18-9-7-12/h2,4,6-10,14,16-17H,3,5H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of human placental aromatase |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50097366

(4-((1H-imidazol-1-yl)methyl)-1-nitro-4aH-xanthen-9...)Show SMILES [O-][N+](=O)c1ccc(Cn2ccnc2)c2oc3ccccc3c(=O)c12 Show InChI InChI=1S/C17H11N3O4/c21-16-12-3-1-2-4-14(12)24-17-11(9-19-8-7-18-10-19)5-6-13(15(16)17)20(22)23/h1-8,10H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of human placental aromatase in presence of [1beta,2beta-3H] testosterone by Thompson and Siiteri method |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50075009
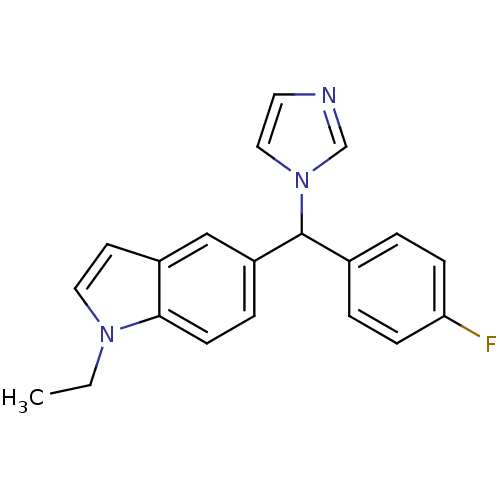
(1-Ethyl-5-[(4-fluoro-phenyl)-imidazol-1-yl-methyl]...)Show InChI InChI=1S/C20H18FN3/c1-2-23-11-9-16-13-17(5-8-19(16)23)20(24-12-10-22-14-24)15-3-6-18(21)7-4-15/h3-14,20H,2H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123024
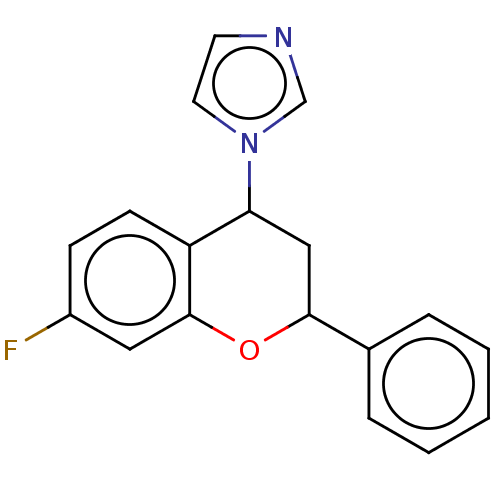
(CHEMBL3623227)Show InChI InChI=1S/C18H15FN2O/c19-14-6-7-15-16(21-9-8-20-12-21)11-17(22-18(15)10-14)13-4-2-1-3-5-13/h1-10,12,16-17H,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of human placental aromatase using [1,2,6,7-3H] androstenedione as substrate |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123152
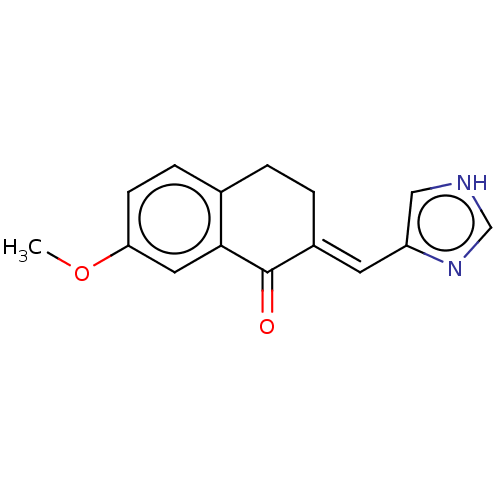
(CHEMBL3219600)Show InChI InChI=1S/C15H14N2O2/c1-19-13-5-4-10-2-3-11(15(18)14(10)7-13)6-12-8-16-9-17-12/h4-9H,2-3H2,1H3,(H,16,17)/b11-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50097373
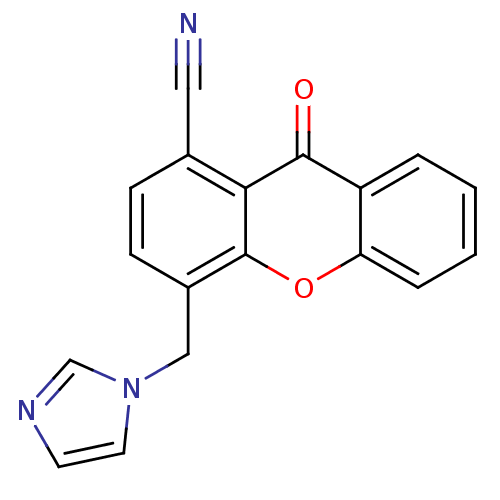
(4-((1H-imidazol-1-yl)methyl)-9-oxo-9H-xanthene-1-c...)Show InChI InChI=1S/C18H11N3O2/c19-9-12-5-6-13(10-21-8-7-20-11-21)18-16(12)17(22)14-3-1-2-4-15(14)23-18/h1-8,11H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of human placental aromatase in presence of [1beta,2beta-3H] testosterone by Thompson and Siiteri method |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9475
(4-(1H-Imidazol-1-ylmethyl)-7-phenoxy-2H-chromen-2-...)Show InChI InChI=1S/C19H14N2O3/c22-19-10-14(12-21-9-8-20-13-21)17-7-6-16(11-18(17)24-19)23-15-4-2-1-3-5-15/h1-11,13H,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of human placental aromatase in presence of [1beta,2beta-3H] testosterone by Thompson and Siiteri method |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123153
(CHEMBL3623225)Show InChI InChI=1S/C20H19N3/c1-2-22-14-18(17-10-6-7-11-19(17)22)20(23-13-12-21-15-23)16-8-4-3-5-9-16/h3-15,20H,2H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123016

(CHEMBL3623214)Show SMILES [H][C@@]12C[C@@H](C(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)C=C[C@]12C)n1ccnc1 |r,c:22,t:18| Show InChI InChI=1S/C22H26N2O2/c1-21-7-5-15(25)11-14(21)3-4-16-17(21)6-8-22(2)18(16)12-19(20(22)26)24-10-9-23-13-24/h5,7,9-11,13,16-19H,3-4,6,8,12H2,1-2H3/t16-,17+,18+,19+,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Rattus norvegicus) | BDBM50049763
(2-[1-(3H-Imidazol-4-yl)-meth-(E)-ylidene]-3,4-dihy...)Show InChI InChI=1S/C14H12N2O/c17-14-11(7-12-8-15-9-16-12)6-5-10-3-1-2-4-13(10)14/h1-4,7-9H,5-6H2,(H,15,16) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of rat ovarian aromatase |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123012
(CHEMBL3623217)Show SMILES [H][C@@]12C[C@H](C#N)[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=C(Sc3ccccc3)C(=O)CC[C@]12C |r,c:20| Show InChI InChI=1S/C26H31NO2S/c1-25-13-11-22(28)23(30-17-6-4-3-5-7-17)20(25)9-8-18-19(25)10-12-26(2)21(18)14-16(15-27)24(26)29/h3-7,16,18-19,21,24,29H,8-14H2,1-2H3/t16-,18-,19+,21+,24+,25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123017

(CHEMBL3623213)Show SMILES [H][C@@]12C[C@@H](C(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C)n1ccnc1 |r,t:18| Show InChI InChI=1S/C22H28N2O2/c1-21-7-5-15(25)11-14(21)3-4-16-17(21)6-8-22(2)18(16)12-19(20(22)26)24-10-9-23-13-24/h9-11,13,16-19H,3-8,12H2,1-2H3/t16-,17+,18+,19+,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123023

(CHEMBL3623226)Show InChI InChI=1S/C17H12FN3O/c18-14-7-5-12(6-8-14)17(21-11-19-10-20-21)16-9-13-3-1-2-4-15(13)22-16/h1-11,17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of human placental aromatase |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123019

(CHEMBL3621226)Show SMILES [H][C@@]12C=CC3=CC(=O)CC[C@]3(C)[C@@]1([H])CC[C@@](C)([C@H]2CC#N)C(C)=O |r,c:2,t:4| Show InChI InChI=1S/C20H25NO2/c1-13(22)19(2)10-7-17-16(18(19)8-11-21)5-4-14-12-15(23)6-9-20(14,17)3/h4-5,12,16-18H,6-10H2,1-3H3/t16-,17+,18+,19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123020
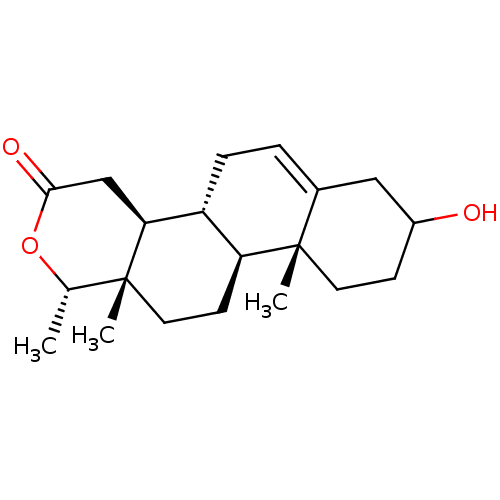
(CHEMBL3623209)Show SMILES [H][C@@]12CC=C3CC(O)CC[C@]3(C)[C@@]1([H])CC[C@]1(C)[C@H](C)OC(=O)C[C@@]21[H] |r,t:3| Show InChI InChI=1S/C20H30O3/c1-12-19(2)9-7-16-15(17(19)11-18(22)23-12)5-4-13-10-14(21)6-8-20(13,16)3/h4,12,14-17,21H,5-11H2,1-3H3/t12-,14?,15+,16-,17-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Rattus norvegicus) | BDBM50123022

(CHEMBL169251)Show InChI InChI=1S/C14H12N2O/c17-14-11(7-12-8-15-9-16-12)6-5-10-3-1-2-4-13(10)14/h1-4,7-9H,5-6H2,(H,15,16)/b11-7- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of rat ovarian aromatase |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123021

(CHEMBL3623208)Show SMILES [H][C@@]12CCC3=CC(=O)CC[C@]3(C)[C@@]1([H])CC[C@@](C)([C@H]2CC#N)C(C)=O |r,t:4| Show InChI InChI=1S/C20H27NO2/c1-13(22)19(2)10-7-17-16(18(19)8-11-21)5-4-14-12-15(23)6-9-20(14,17)3/h12,16-18H,4-10H2,1-3H3/t16-,17+,18+,19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123151
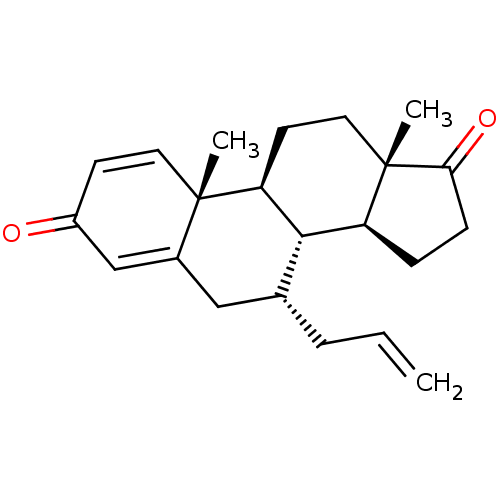
(CHEMBL3623223)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])[C@H](CC=C)CC2=CC(=O)C=C[C@]12C |r,c:25,t:21| Show InChI InChI=1S/C22H28O2/c1-4-5-14-12-15-13-16(23)8-10-21(15,2)18-9-11-22(3)17(20(14)18)6-7-19(22)24/h4,8,10,13-14,17-18,20H,1,5-7,9,11-12H2,2-3H3/t14-,17+,18+,20+,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123030
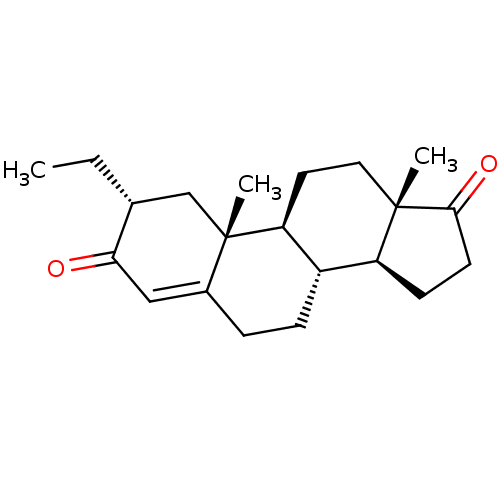
(CHEMBL3623219)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)[C@H](CC)C[C@]12C |r,t:18| Show InChI InChI=1S/C21H30O2/c1-4-13-12-21(3)14(11-18(13)22)5-6-15-16-7-8-19(23)20(16,2)10-9-17(15)21/h11,13,15-17H,4-10,12H2,1-3H3/t13-,15+,16+,17+,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM7461
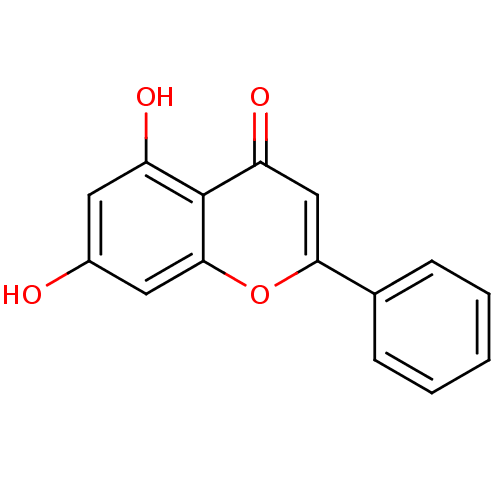
(5,7-dihydroxy-2-phenyl-4H-chromen-4-one | 5,7-dihy...)Show InChI InChI=1S/C15H10O4/c16-10-6-11(17)15-12(18)8-13(19-14(15)7-10)9-4-2-1-3-5-9/h1-8,16-17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10027
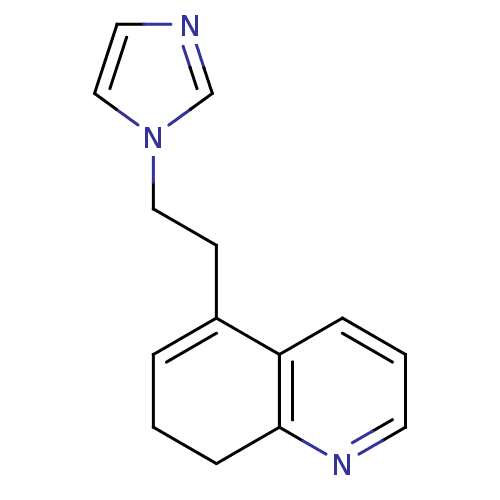
(5-[2-(1H-imidazol-1-yl)ethyl]-7,8-dihydroquinoline...)Show InChI InChI=1S/C14H15N3/c1-3-12(6-9-17-10-8-15-11-17)13-4-2-7-16-14(13)5-1/h2-4,7-8,10-11H,1,5-6,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123014

(CHEMBL3623215)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])C(=O)c3[nH]ncc3C[C@]12C |r| Show InChI InChI=1S/C20H28N2O2/c1-19-8-7-14-12(13(19)5-6-16(19)23)3-4-15-18(24)17-11(10-21-22-17)9-20(14,15)2/h10,12-16,23H,3-9H2,1-2H3,(H,21,22)/t12-,13-,14-,15-,16-,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123011

(CHEMBL3623218)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)[C@H](F)C[C@]12C |r,t:18| Show InChI InChI=1S/C19H25FO2/c1-18-8-7-14-12(13(18)5-6-17(18)22)4-3-11-9-16(21)15(20)10-19(11,14)2/h9,12-15H,3-8,10H2,1-2H3/t12-,13-,14-,15+,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123013
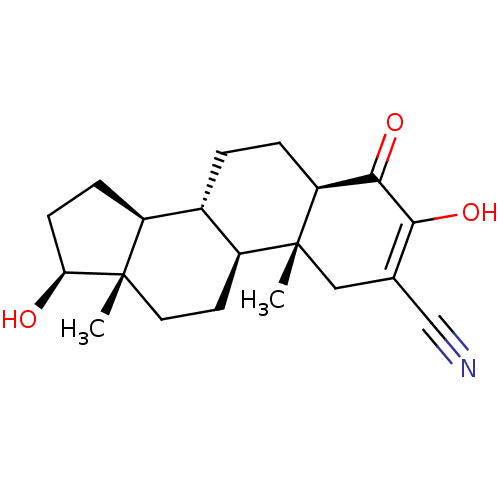
(CHEMBL3623216)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])C(=O)C(O)=C(C[C@]12C)C#N |r,c:23| Show InChI InChI=1S/C20H27NO3/c1-19-8-7-14-12(13(19)5-6-16(19)22)3-4-15-18(24)17(23)11(10-21)9-20(14,15)2/h12-16,22-23H,3-9H2,1-2H3/t12-,13-,14-,15-,16-,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM7458

(5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123027
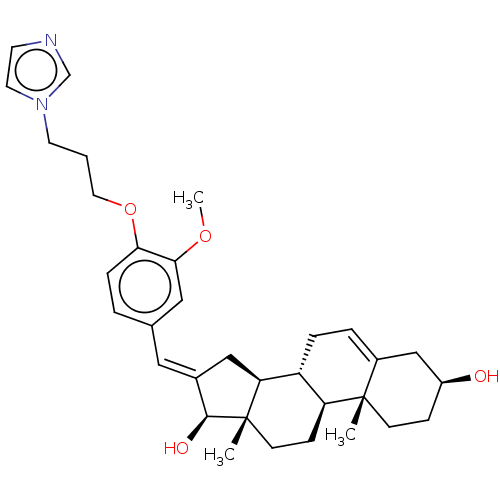
(CHEMBL3623212)Show SMILES [H][C@@]12C\C(=C/c3ccc(OCCCn4ccnc4)c(OC)c3)[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |r,t:37| Show InChI InChI=1S/C33H44N2O4/c1-32-11-9-25(36)20-24(32)6-7-26-27(32)10-12-33(2)28(26)19-23(31(33)37)17-22-5-8-29(30(18-22)38-3)39-16-4-14-35-15-13-34-21-35/h5-6,8,13,15,17-18,21,25-28,31,36-37H,4,7,9-12,14,16,19-20H2,1-3H3/b23-17+/t25-,26+,27-,28-,31-,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of human placental aromatase assessed as conversion of 3H2O from [1beta,2beta-3H] testosterone after 20 mins by scintillation spectrometer... |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM24306

(Letrozole derivative, 40 | {2-bromo-4-[(4-cyanophe...)Show SMILES NS(=O)(=O)Oc1ccc(cc1Br)C(c1ccc(cc1)C#N)n1cncn1 Show InChI InChI=1S/C16H12BrN5O3S/c17-14-7-13(5-6-15(14)25-26(19,23)24)16(22-10-20-9-21-22)12-3-1-11(8-18)2-4-12/h1-7,9-10,16H,(H2,19,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human JEG-3 cells using [6,7-3H]E1S as substrate after 1 hr by scintillation spectrometry |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123155
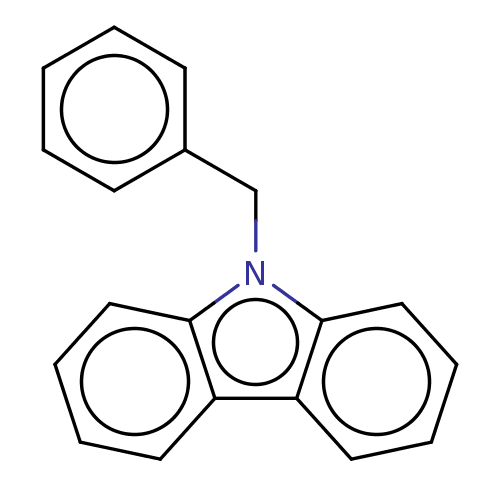
(CHEMBL3623230)Show InChI InChI=1S/C19H15N/c1-2-8-15(9-3-1)14-20-18-12-6-4-10-16(18)17-11-5-7-13-19(17)20/h1-13H,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM13062

((4-{[4-(sulfamoyloxy)phenyl](1H-1,2,4-triazol-1-yl...)Show SMILES NS(=O)(=O)Oc1ccc(cc1)C(c1ccc(OS(N)(=O)=O)cc1)n1cncn1 Show InChI InChI=1S/C15H15N5O6S2/c16-27(21,22)25-13-5-1-11(2-6-13)15(20-10-18-9-19-20)12-3-7-14(8-4-12)26-28(17,23)24/h1-10,15H,(H2,16,21,22)(H2,17,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human JEG-3 cells using [1beta-3H]androstenedione as substrate after 1 hr by scintillation spectrometry |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123015
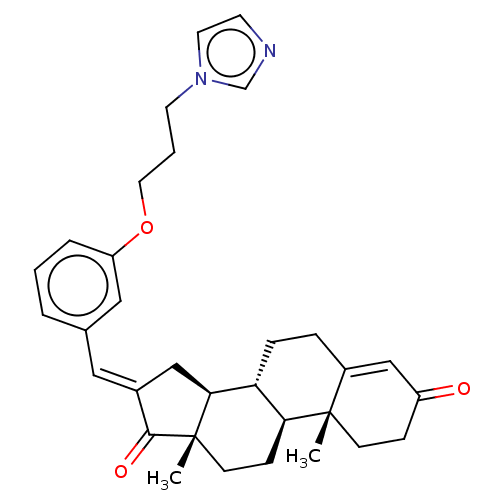
(CHEMBL2282050)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1C\C(=C/c1cccc(OCCCn3ccnc3)c1)C2=O |r,t:8| Show InChI InChI=1S/C32H38N2O3/c1-31-11-9-25(35)20-24(31)7-8-27-28(31)10-12-32(2)29(27)19-23(30(32)36)17-22-5-3-6-26(18-22)37-16-4-14-34-15-13-33-21-34/h3,5-6,13,15,17-18,20-21,27-29H,4,7-12,14,16,19H2,1-2H3/b23-17+/t27-,28+,29+,31+,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Rattus norvegicus) | BDBM50009605
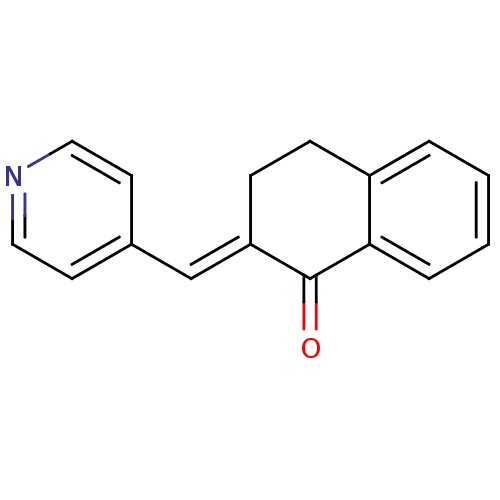
(2-(pyridin-4-ylmethylene)-3,4-dihydronaphthalen-1(...)Show InChI InChI=1S/C16H13NO/c18-16-14(11-12-7-9-17-10-8-12)6-5-13-3-1-2-4-15(13)16/h1-4,7-11H,5-6H2/b14-11+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of rat ovarian aromatase |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123018

(CHEMBL2003274)Show SMILES [H][C@@]12C\C(=C/c3ccncc3)C(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |t:26| Show InChI InChI=1S/C25H29NO2/c1-24-9-5-19(27)15-18(24)3-4-20-21(24)6-10-25(2)22(20)14-17(23(25)28)13-16-7-11-26-12-8-16/h7-8,11-13,15,20-22H,3-6,9-10,14H2,1-2H3/b17-13+/t20-,21+,22+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123154
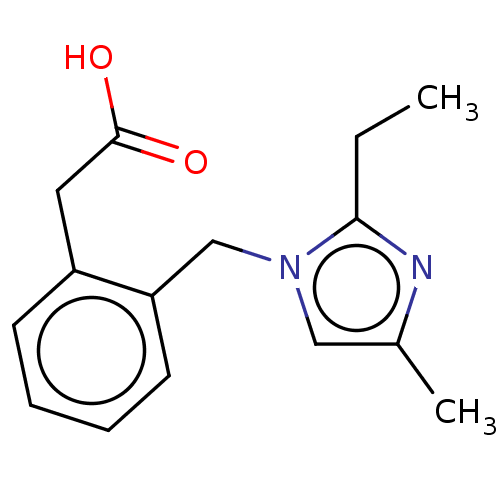
(CHEMBL3623229)Show InChI InChI=1S/C15H18N2O2/c1-3-14-16-11(2)9-17(14)10-13-7-5-4-6-12(13)8-15(18)19/h4-7,9H,3,8,10H2,1-2H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50051358
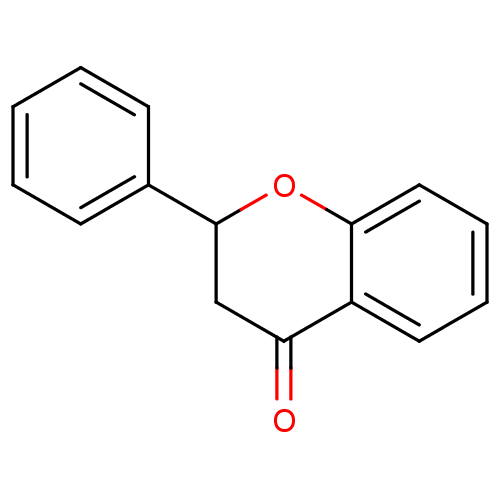
(2,3-Dihydroflavone | 2,3-dihydro-2-phenyl-4H-1-ben...)Show InChI InChI=1S/C15H12O2/c16-13-10-15(11-6-2-1-3-7-11)17-14-9-5-4-8-12(13)14/h1-9,15H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50028962
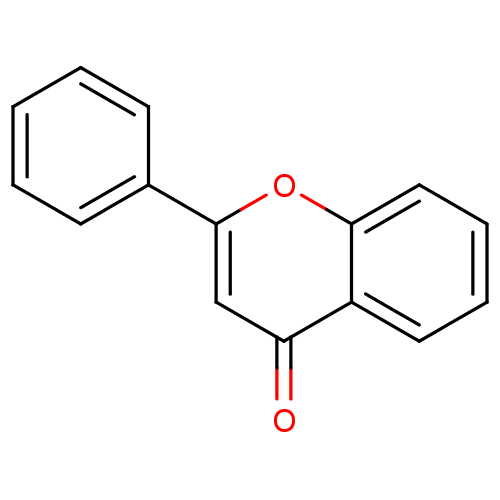
(CHEMBL275638 | flavone)Show InChI InChI=1S/C15H10O2/c16-13-10-15(11-6-2-1-3-7-11)17-14-9-5-4-8-12(13)14/h1-10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM13062

((4-{[4-(sulfamoyloxy)phenyl](1H-1,2,4-triazol-1-yl...)Show SMILES NS(=O)(=O)Oc1ccc(cc1)C(c1ccc(OS(N)(=O)=O)cc1)n1cncn1 Show InChI InChI=1S/C15H15N5O6S2/c16-27(21,22)25-13-5-1-11(2-6-13)15(20-10-18-9-19-20)12-3-7-14(8-4-12)26-28(17,23)24/h1-10,15H,(H2,16,21,22)(H2,17,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human JEG-3 cells using [6,7-3H]E1S as substrate after 1 hr by scintillation spectrometry |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
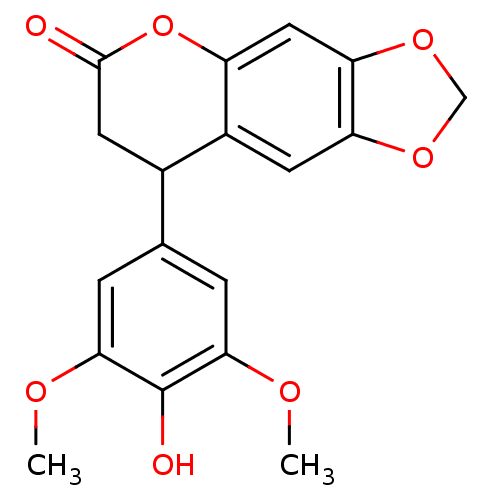
(Homo sapiens (Human)) | BDBM92556
(Neoflavonoid, 8)Show InChI InChI=1S/C18H16O7/c1-21-15-3-9(4-16(22-2)18(15)20)10-6-17(19)25-12-7-14-13(5-11(10)12)23-8-24-14/h3-5,7,10,20H,6,8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) after 30 mins by fluorescence assay |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123029

(CHEMBL3623221)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@]1([H])c3ccc(O)c(NC(=O)c4ccncc4)c3CC[C@@]21[H] |r| Show InChI InChI=1S/C24H26N2O3/c1-24-11-8-16-15-4-6-20(27)22(26-23(29)14-9-12-25-13-10-14)18(15)3-2-17(16)19(24)5-7-21(24)28/h4,6,9-10,12-13,16-17,19,27H,2-3,5,7-8,11H2,1H3,(H,26,29)/t16-,17-,19+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of human placental aromatase assessed as conversion of 3H2O from [1beta-3H]AD after 20 mins by Siiteri and Thompson method |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123028

(CHEMBL3623220)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@]1([H])c3cc(NC(=O)c4ccncc4)c(O)cc3CC[C@@]21[H] |r| Show InChI InChI=1S/C24H26N2O3/c1-24-9-6-16-17(19(24)4-5-22(24)28)3-2-15-12-21(27)20(13-18(15)16)26-23(29)14-7-10-25-11-8-14/h7-8,10-13,16-17,19,27H,2-6,9H2,1H3,(H,26,29)/t16-,17+,19-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of human placental aromatase assessed as conversion of 3H2O from [1beta-3H]AD after 20 mins by Siiteri and Thompson method |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
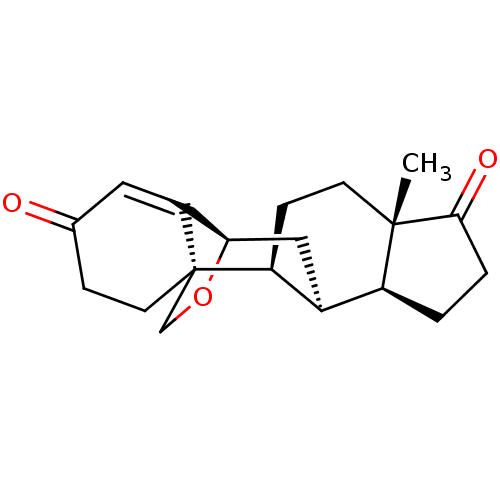
(Homo sapiens (Human)) | BDBM50123150
(CHEMBL3623222)Show SMILES [H][C@]12C[C@@]3([H])[C@]4([H])CCC(=O)[C@@]4(C)CC[C@]3([H])[C@@]3(CO1)CCC(=O)C=C23 |r,t:27| Show InChI InChI=1S/C19H24O3/c1-18-6-5-14-12(13(18)2-3-17(18)21)9-16-15-8-11(20)4-7-19(14,15)10-22-16/h8,12-14,16H,2-7,9-10H2,1H3/t12-,13-,14-,16+,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
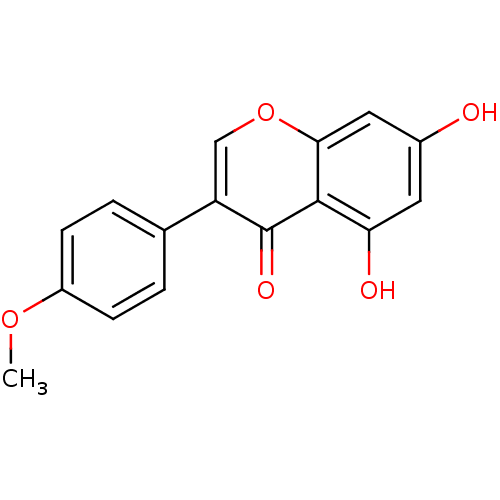
(Homo sapiens (Human)) | BDBM9461
(5,7-dihydroxy-3-(4-methoxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C16H12O5/c1-20-11-4-2-9(3-5-11)12-8-21-14-7-10(17)6-13(18)15(14)16(12)19/h2-8,17-18H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.13E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9454
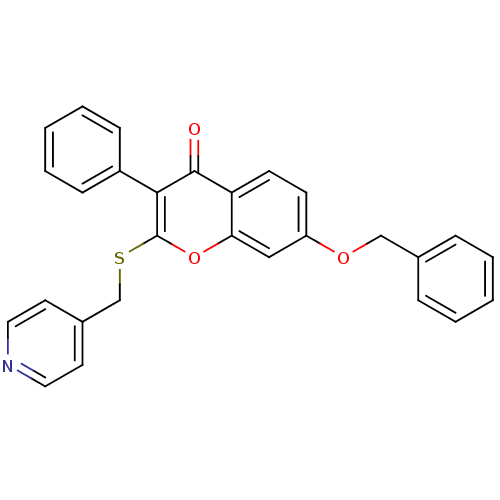
(7-(benzyloxy)-3-phenyl-2-[(pyridin-4-ylmethyl)sulf...)Show SMILES O=c1c(-c2ccccc2)c(SCc2ccncc2)oc2cc(OCc3ccccc3)ccc12 Show InChI InChI=1S/C28H21NO3S/c30-27-24-12-11-23(31-18-20-7-3-1-4-8-20)17-25(24)32-28(26(27)22-9-5-2-6-10-22)33-19-21-13-15-29-16-14-21/h1-17H,18-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of human placental aromatase assessed as conversion of [1beta-3H]androst-4-ene-3,17-dione to estrone after 15 mins by scintillation counti... |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data