

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactoperoxidase (Homo sapiens (Human)) | BDBM50275890 (CHEMBL457523 | ethyl 2,3-dihydro-3-methyl-2-seleno...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of lactoperoxidase (unknown origin)-catalyzed iodination of L-tyrosine assessed as 3,5-diiodo-L-tyrosine formation by HPLC | J Med Chem 51: 7313-7 (2009) Article DOI: 10.1021/jm800894m BindingDB Entry DOI: 10.7270/Q2Z31ZGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoperoxidase (Homo sapiens (Human)) | BDBM50275889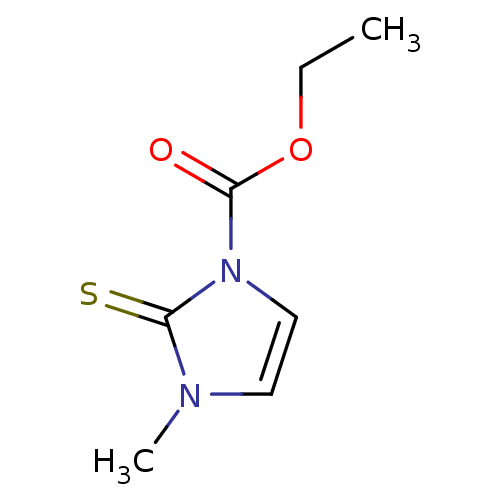 (CHEMBL508102 | carbimazole) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of lactoperoxidase (unknown origin)-catalyzed iodination of L-tyrosine assessed as 3,5-diiodo-L-tyrosine formation by HPLC | J Med Chem 51: 7313-7 (2009) Article DOI: 10.1021/jm800894m BindingDB Entry DOI: 10.7270/Q2Z31ZGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoperoxidase (Homo sapiens (Human)) | BDBM50275891 (CHEMBL444464 | methyl 3-methyl-2-thioxo-2,3-dihydr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of lactoperoxidase (unknown origin)-catalyzed iodination of L-tyrosine assessed as 3,5-diiodo-L-tyrosine formation by HPLC | J Med Chem 51: 7313-7 (2009) Article DOI: 10.1021/jm800894m BindingDB Entry DOI: 10.7270/Q2Z31ZGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoperoxidase (Homo sapiens (Human)) | BDBM50241361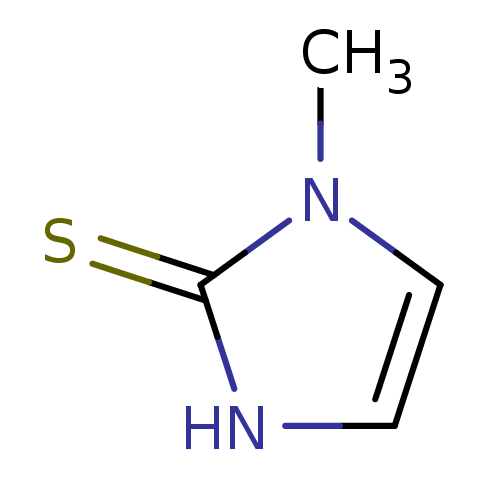 (CHEMBL1515 | METHIMAZOLE | US9138393, Methimazole ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of lactoperoxidase (unknown origin)-catalyzed iodination of L-tyrosine assessed as 3,5-diiodo-L-tyrosine formation by HPLC | J Med Chem 51: 7313-7 (2009) Article DOI: 10.1021/jm800894m BindingDB Entry DOI: 10.7270/Q2Z31ZGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoperoxidase (Homo sapiens (Human)) | BDBM50275895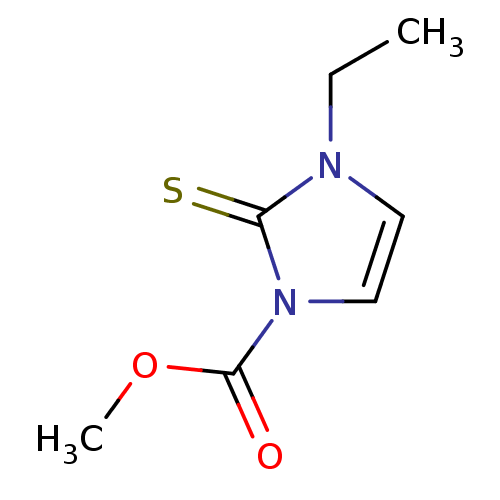 (CHEMBL469530 | methyl 3-ethyl-2-thioxo-2,3-dihydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of lactoperoxidase (unknown origin)-catalyzed iodination of L-tyrosine assessed as 3,5-diiodo-L-tyrosine formation by HPLC | J Med Chem 51: 7313-7 (2009) Article DOI: 10.1021/jm800894m BindingDB Entry DOI: 10.7270/Q2Z31ZGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoperoxidase (Homo sapiens (Human)) | BDBM50275892 (CHEMBL507966 | methyl 3-methyl-2-selenoxo-2,3-dihy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of lactoperoxidase (unknown origin)-catalyzed iodination of L-tyrosine assessed as 3,5-diiodo-L-tyrosine formation by HPLC | J Med Chem 51: 7313-7 (2009) Article DOI: 10.1021/jm800894m BindingDB Entry DOI: 10.7270/Q2Z31ZGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoperoxidase (Homo sapiens (Human)) | BDBM50275893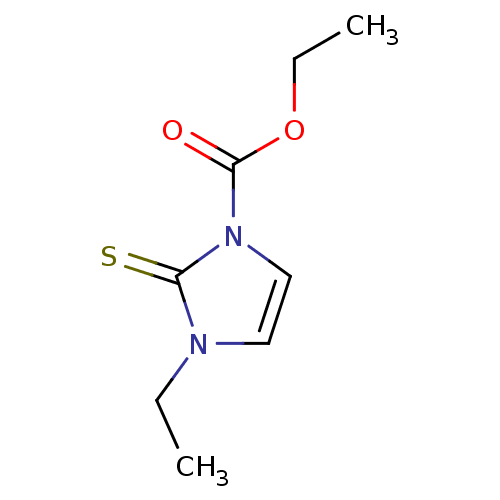 (CHEMBL469529 | ethyl 3-ethyl-2-thioxo-2,3-dihydro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of lactoperoxidase (unknown origin)-catalyzed iodination of L-tyrosine assessed as 3,5-diiodo-L-tyrosine formation by HPLC | J Med Chem 51: 7313-7 (2009) Article DOI: 10.1021/jm800894m BindingDB Entry DOI: 10.7270/Q2Z31ZGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoperoxidase (Homo sapiens (Human)) | BDBM50275894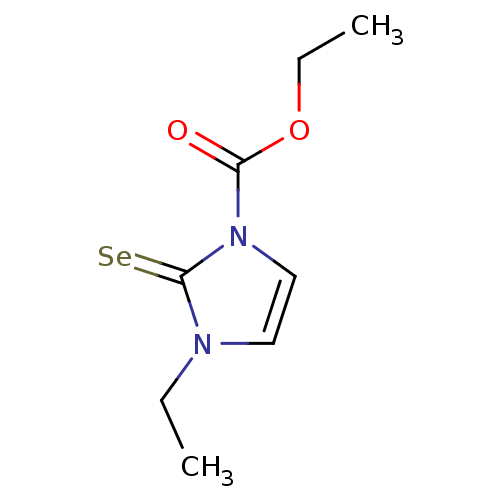 (CHEMBL446332 | ethyl 3-ethyl-2-selenoxo-2,3-dihydr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of lactoperoxidase (unknown origin)-catalyzed iodination of L-tyrosine assessed as 3,5-diiodo-L-tyrosine formation by HPLC | J Med Chem 51: 7313-7 (2009) Article DOI: 10.1021/jm800894m BindingDB Entry DOI: 10.7270/Q2Z31ZGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoperoxidase (Homo sapiens (Human)) | BDBM50275944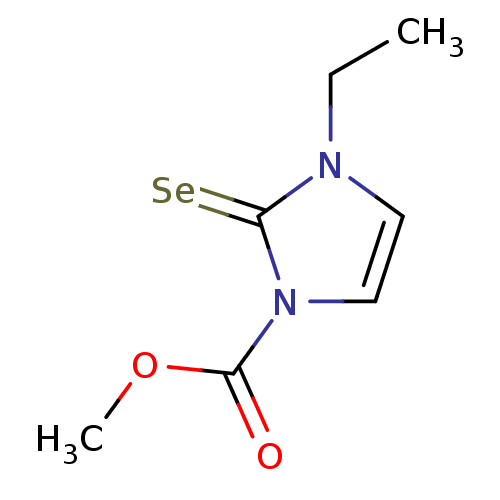 (CHEMBL459114 | methyl 3-ethyl-2-selenoxo-2,3-dihyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of lactoperoxidase (unknown origin)-catalyzed iodination of L-tyrosine assessed as 3,5-diiodo-L-tyrosine formation by HPLC | J Med Chem 51: 7313-7 (2009) Article DOI: 10.1021/jm800894m BindingDB Entry DOI: 10.7270/Q2Z31ZGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||