 Found 34 hits Enz. Inhib. hit(s) with all data for entry = 50047686
Found 34 hits Enz. Inhib. hit(s) with all data for entry = 50047686 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50183060
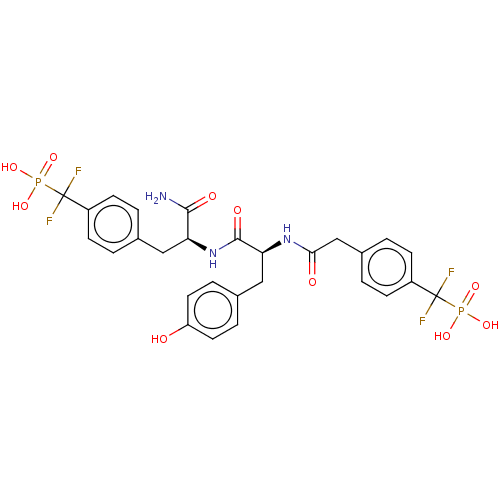
(CHEMBL3818452)Show SMILES NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O |r| Show InChI InChI=1S/C28H29F4N3O10P2/c29-27(30,46(40,41)42)19-7-1-16(2-8-19)13-22(25(33)38)35-26(39)23(14-17-5-11-21(36)12-6-17)34-24(37)15-18-3-9-20(10-4-18)28(31,32)47(43,44)45/h1-12,22-23,36H,13-15H2,(H2,33,38)(H,34,37)(H,35,39)(H2,40,41,42)(H2,43,44,45)/t22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B catalytic domain (1 to 321 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using p-Nitrophenyl phosphat... |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50183060

(CHEMBL3818452)Show SMILES NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O |r| Show InChI InChI=1S/C28H29F4N3O10P2/c29-27(30,46(40,41)42)19-7-1-16(2-8-19)13-22(25(33)38)35-26(39)23(14-17-5-11-21(36)12-6-17)34-24(37)15-18-3-9-20(10-4-18)28(31,32)47(43,44)45/h1-12,22-23,36H,13-15H2,(H2,33,38)(H,34,37)(H,35,39)(H2,40,41,42)(H2,43,44,45)/t22-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP (unknown origin) |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50183057
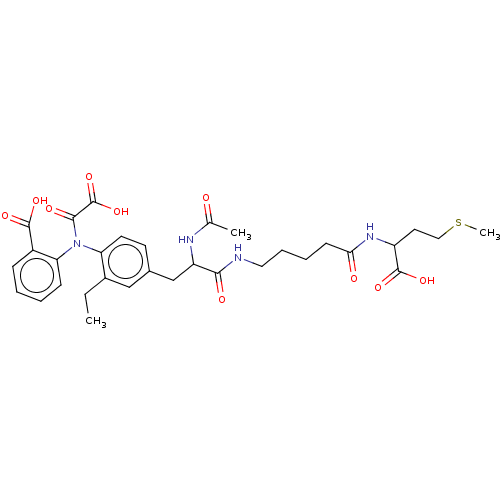
(CHEMBL3818765)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCC(=O)NC(CCSC)C(O)=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C32H40N4O10S/c1-4-21-17-20(12-13-25(21)36(29(40)32(45)46)26-10-6-5-9-22(26)30(41)42)18-24(34-19(2)37)28(39)33-15-8-7-11-27(38)35-23(31(43)44)14-16-47-3/h5-6,9-10,12-13,17,23-24H,4,7-8,11,14-16,18H2,1-3H3,(H,33,39)(H,34,37)(H,35,38)(H,41,42)(H,43,44)(H,45,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) using p-nitrophenyl phosphate as substrate by colorimetric method |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131545
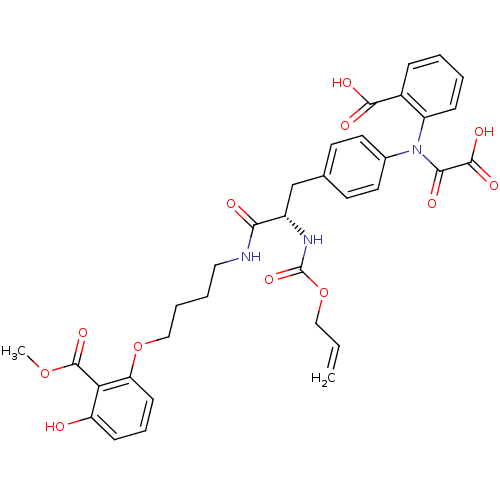
((S)-2-(N-(4-(2-(allyloxycarbonylamino)-3-(4-(3-hyd...)Show SMILES COC(=O)c1c(O)cccc1OCCCCNC(=O)[C@H](Cc1ccc(cc1)N(C(=O)C(O)=O)c1ccccc1C(O)=O)NC(=O)OCC=C Show InChI InChI=1S/C34H35N3O12/c1-3-18-49-34(46)36-24(29(39)35-17-6-7-19-48-27-12-8-11-26(38)28(27)33(45)47-2)20-21-13-15-22(16-14-21)37(30(40)32(43)44)25-10-5-4-9-23(25)31(41)42/h3-5,8-16,24,38H,1,6-7,17-20H2,2H3,(H,35,39)(H,36,46)(H,41,42)(H,43,44)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 288 residues) expressed in Escherichia coli BL21 (DE3) cells using p-nitrophenyl phosphate as substrate monitered eve... |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50183059

(CHEMBL3818451)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(C(=O)C(=O)Nc2ccccc2C(O)=O)c(\C=C\C(N)=O)c1)NS(C)(=O)=O |r| Show InChI InChI=1S/C27H32N4O8S/c1-3-4-7-14-29-25(34)22(31-40(2,38)39)16-17-10-12-19(18(15-17)11-13-23(28)32)24(33)26(35)30-21-9-6-5-8-20(21)27(36)37/h5-6,8-13,15,22,31H,3-4,7,14,16H2,1-2H3,(H2,28,32)(H,29,34)(H,30,35)(H,36,37)/b13-11+/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP (unknown origin) using p-nitrophenyl phosphate as substrate by colorimetric method |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50183059

(CHEMBL3818451)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(C(=O)C(=O)Nc2ccccc2C(O)=O)c(\C=C\C(N)=O)c1)NS(C)(=O)=O |r| Show InChI InChI=1S/C27H32N4O8S/c1-3-4-7-14-29-25(34)22(31-40(2,38)39)16-17-10-12-19(18(15-17)11-13-23(28)32)24(33)26(35)30-21-9-6-5-8-20(21)27(36)37/h5-6,8-13,15,22,31H,3-4,7,14,16H2,1-2H3,(H2,28,32)(H,29,34)(H,30,35)(H,36,37)/b13-11+/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 288 residues) expressed in Escherichia coli BL21 (DE3) cells using p-nitrophenyl phosphate as substrate by colorimetr... |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118750
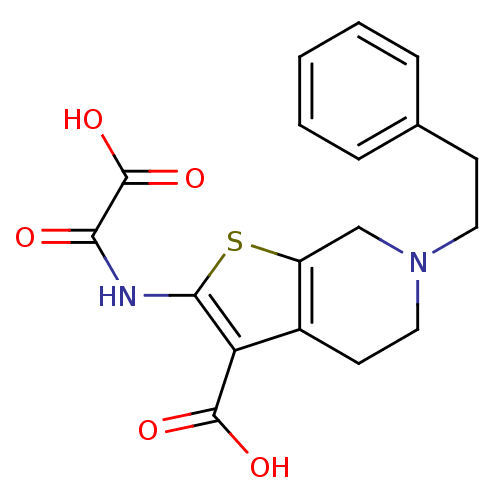
(2-(Oxalyl-amino)-6-phenethyl-4,5,6,7-tetrahydro-th...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCc3ccccc3)CCc2c1C(O)=O Show InChI InChI=1S/C18H18N2O5S/c21-15(18(24)25)19-16-14(17(22)23)12-7-9-20(10-13(12)26-16)8-6-11-4-2-1-3-5-11/h1-5H,6-10H2,(H,19,21)(H,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B (1 to 321 residues) catalytic domain expressed in Escherichia coli BL21 (DE3) cells using p-nitrophenyl phospha... |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50183057

(CHEMBL3818765)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCC(=O)NC(CCSC)C(O)=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C32H40N4O10S/c1-4-21-17-20(12-13-25(21)36(29(40)32(45)46)26-10-6-5-9-22(26)30(41)42)18-24(34-19(2)37)28(39)33-15-8-7-11-27(38)35-23(31(43)44)14-16-47-3/h5-6,9-10,12-13,17,23-24H,4,7-8,11,14-16,18H2,1-3H3,(H,33,39)(H,34,37)(H,35,38)(H,41,42)(H,43,44)(H,45,46) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP (unknown origin) using p-nitrophenyl phosphate as substrate by colorimetric method |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50131545

((S)-2-(N-(4-(2-(allyloxycarbonylamino)-3-(4-(3-hyd...)Show SMILES COC(=O)c1c(O)cccc1OCCCCNC(=O)[C@H](Cc1ccc(cc1)N(C(=O)C(O)=O)c1ccccc1C(O)=O)NC(=O)OCC=C Show InChI InChI=1S/C34H35N3O12/c1-3-18-49-34(46)36-24(29(39)35-17-6-7-19-48-27-12-8-11-26(38)28(27)33(45)47-2)20-21-13-15-22(16-14-21)37(30(40)32(43)44)25-10-5-4-9-23(25)31(41)42/h3-5,8-16,24,38H,1,6-7,17-20H2,2H3,(H,35,39)(H,36,46)(H,41,42)(H,43,44)/t24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP (unknown origin) |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50118750

(2-(Oxalyl-amino)-6-phenethyl-4,5,6,7-tetrahydro-th...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCc3ccccc3)CCc2c1C(O)=O Show InChI InChI=1S/C18H18N2O5S/c21-15(18(24)25)19-16-14(17(22)23)12-7-9-20(10-13(12)26-16)8-6-11-4-2-1-3-5-11/h1-5H,6-10H2,(H,19,21)(H,22,23)(H,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant TCPTP (unknown origin) at pH 7 |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308851
((S)-2-(4-(2-acetamido-3-(4-(3-hydroxy-2-(methoxyca...)Show SMILES COC(=O)c1c(O)cccc1OCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(O)c1)NC(C)=O |r| Show InChI InChI=1S/C25H30N2O10/c1-15(28)27-17(12-16-8-9-20(19(30)13-16)37-14-22(31)32)24(33)26-10-3-4-11-36-21-7-5-6-18(29)23(21)25(34)35-2/h5-9,13,17,29-30H,3-4,10-12,14H2,1-2H3,(H,26,33)(H,27,28)(H,31,32)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) using p-nitrophenyl phosphate as substrate by colorimetric method |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50308851

((S)-2-(4-(2-acetamido-3-(4-(3-hydroxy-2-(methoxyca...)Show SMILES COC(=O)c1c(O)cccc1OCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(O)c1)NC(C)=O |r| Show InChI InChI=1S/C25H30N2O10/c1-15(28)27-17(12-16-8-9-20(19(30)13-16)37-14-22(31)32)24(33)26-10-3-4-11-36-21-7-5-6-18(29)23(21)25(34)35-2/h5-9,13,17,29-30H,3-4,10-12,14H2,1-2H3,(H,26,33)(H,27,28)(H,31,32)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP (unknown origin) using p-nitrophenyl phosphate as substrate by colorimetric method |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13599
(3-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES OP(O)(=O)c1cccc(c1)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H29F2N3O6P2/c35-34(36,47(43,44)45)29-19-15-25(16-20-29)23-33(28-8-2-1-3-9-28,39-32-12-5-4-11-31(32)37-38-39)22-24-13-17-26(18-14-24)27-7-6-10-30(21-27)46(40,41)42/h1-21H,22-23H2,(H2,40,41,42)(H2,43,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of flag-tagged PTP1B (1 to 321 residues) (unknown origin) expressed in bacterial expression system by UV/Vis spectrophotometry |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13599

(3-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES OP(O)(=O)c1cccc(c1)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H29F2N3O6P2/c35-34(36,47(43,44)45)29-19-15-25(16-20-29)23-33(28-8-2-1-3-9-28,39-32-12-5-4-11-31(32)37-38-39)22-24-13-17-26(18-14-24)27-7-6-10-30(21-27)46(40,41)42/h1-21H,22-23H2,(H2,40,41,42)(H2,43,44,45) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP (unknown origin) by UV/Vis spectrophotometry |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of flag-tagged PTP1B (1 to 321 residues) (unknown origin) expressed in bacterial expression system by UV/Vis spectrophotometry |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50183065
(CHEMBL541953)Show SMILES CC(=O)N[C@H](Cc1ccc(N(C(=O)C(O)=O)c2ccccc2C(O)=O)c2ccccc12)C(=O)NCCCCCOc1cc2ccccc2cc1C(O)=O |r| Show InChI InChI=1S/C40H37N3O10/c1-24(44)42-32(36(45)41-19-9-2-10-20-53-35-23-26-12-4-3-11-25(26)21-31(35)39(49)50)22-27-17-18-34(29-14-6-5-13-28(27)29)43(37(46)40(51)52)33-16-8-7-15-30(33)38(47)48/h3-8,11-18,21,23,32H,2,9-10,19-20,22H2,1H3,(H,41,45)(H,42,44)(H,47,48)(H,49,50)(H,51,52)/t32-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 288 residues) expressed in Escherichia coli BL21 (DE3) cells using p-nitrophenyl phosphate as substrate monitered eve... |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP (unknown origin) by UV/Vis spectrophotometry |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13814

(({4-[(4E)-2-(1H-1,2,3-benzotriazol-1-yl)-2-[4-(met...)Show SMILES COC(=O)c1ccc(cc1)C(C\C=C\c1ccccc1)(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)n1nnc2ccccc12 Show InChI InChI=1S/C32H28F2N3O5P/c1-42-30(38)25-15-19-26(20-16-25)31(21-7-10-23-8-3-2-4-9-23,37-29-12-6-5-11-28(29)35-36-37)22-24-13-17-27(18-14-24)32(33,34)43(39,40)41/h2-20H,21-22H2,1H3,(H2,39,40,41)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of flag-tagged human PTP1B (1 to 298 residues) catalytic domain expressed in Escherichia coli BL21 cells using fluorescein diphosphate as ... |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50183065

(CHEMBL541953)Show SMILES CC(=O)N[C@H](Cc1ccc(N(C(=O)C(O)=O)c2ccccc2C(O)=O)c2ccccc12)C(=O)NCCCCCOc1cc2ccccc2cc1C(O)=O |r| Show InChI InChI=1S/C40H37N3O10/c1-24(44)42-32(36(45)41-19-9-2-10-20-53-35-23-26-12-4-3-11-25(26)21-31(35)39(49)50)22-27-17-18-34(29-14-6-5-13-28(27)29)43(37(46)40(51)52)33-16-8-7-15-30(33)38(47)48/h3-8,11-18,21,23,32H,2,9-10,19-20,22H2,1H3,(H,41,45)(H,42,44)(H,47,48)(H,49,50)(H,51,52)/t32-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP (unknown origin) |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13814

(({4-[(4E)-2-(1H-1,2,3-benzotriazol-1-yl)-2-[4-(met...)Show SMILES COC(=O)c1ccc(cc1)C(C\C=C\c1ccccc1)(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)n1nnc2ccccc12 Show InChI InChI=1S/C32H28F2N3O5P/c1-42-30(38)25-15-19-26(20-16-25)31(21-7-10-23-8-3-2-4-9-23,37-29-12-6-5-11-28(29)35-36-37)22-24-13-17-27(18-14-24)32(33,34)43(39,40)41/h2-20H,21-22H2,1H3,(H2,39,40,41)/b10-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human TCPTP (1 to 296 residues) catalytic domain expressed in Escherichia coli BL21 cells using fluorescein diphosphate as substrate by... |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13816
([difluoro({4-[3-(4-fluorophenyl)-2-[4-(3-methyl-1,...)Show SMILES Cc1noc(n1)-c1ccc(cc1)C(C\C=C\c1ccccc1)(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)C(=O)c1ccc(F)cc1 Show InChI InChI=1S/C34H28F3N2O5P/c1-23-38-32(44-39-23)27-11-17-28(18-12-27)33(21-5-8-24-6-3-2-4-7-24,31(40)26-13-19-30(35)20-14-26)22-25-9-15-29(16-10-25)34(36,37)45(41,42)43/h2-20H,21-22H2,1H3,(H2,41,42,43)/b8-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 163 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of flag-tagged human PTP1B (1 to 298 residues) catalytic domain expressed in Escherichia coli BL21 cells using fluorescein diphosphate as ... |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13997
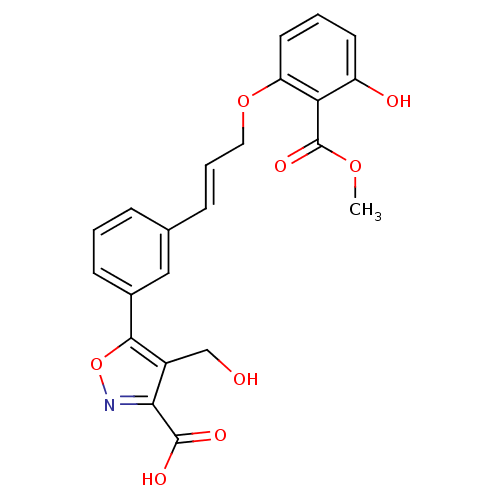
(5-{3-[(1E)-3-[3-hydroxy-2-(methoxycarbonyl)phenoxy...)Show SMILES COC(=O)c1c(O)cccc1OC\C=C\c1cccc(c1)-c1onc(C(O)=O)c1CO Show InChI InChI=1S/C22H19NO8/c1-29-22(28)18-16(25)8-3-9-17(18)30-10-4-6-13-5-2-7-14(11-13)20-15(12-24)19(21(26)27)23-31-20/h2-9,11,24-25H,10,12H2,1H3,(H,26,27)/b6-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50183055
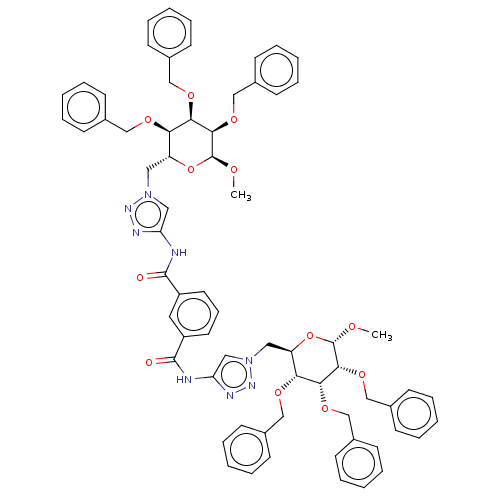
(CHEMBL3818449)Show SMILES CO[C@H]1O[C@H](Cn2cc(NC(=O)c3cccc(c3)C(=O)Nc3cn(C[C@H]4O[C@H](OC)[C@H](OCc5ccccc5)[C@H](OCc5ccccc5)[C@@H]4OCc4ccccc4)nn3)nn2)[C@@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C68H70N8O12/c1-79-67-63(85-45-51-30-17-7-18-31-51)61(83-43-49-26-13-5-14-27-49)59(81-41-47-22-9-3-10-23-47)55(87-67)37-75-39-57(71-73-75)69-65(77)53-34-21-35-54(36-53)66(78)70-58-40-76(74-72-58)38-56-60(82-42-48-24-11-4-12-25-48)62(84-44-50-28-15-6-16-29-50)64(68(80-2)88-56)86-46-52-32-19-8-20-33-52/h3-36,39-40,55-56,59-64,67-68H,37-38,41-46H2,1-2H3,(H,69,77)(H,70,78)/t55-,56-,59-,60-,61-,62-,63-,64-,67+,68+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B catalytic domain using p-nitrophenyl phosphate as substrate by micro plate reader method |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50326508
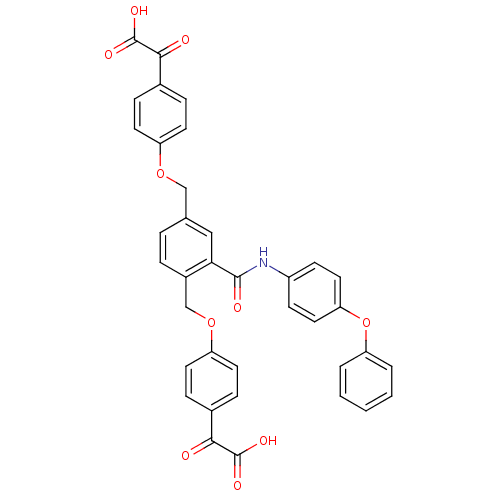
(2,2'-(4,4'-(2-(4-phenoxyphenylcarbamoyl)-1,4-pheny...)Show SMILES OC(=O)C(=O)c1ccc(OCc2ccc(COc3ccc(cc3)C(=O)C(O)=O)c(c2)C(=O)Nc2ccc(Oc3ccccc3)cc2)cc1 Show InChI InChI=1S/C37H27NO10/c39-33(36(42)43)24-8-14-28(15-9-24)46-21-23-6-7-26(22-47-29-16-10-25(11-17-29)34(40)37(44)45)32(20-23)35(41)38-27-12-18-31(19-13-27)48-30-4-2-1-3-5-30/h1-20H,21-22H2,(H,38,41)(H,42,43)(H,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli using p-nitrophenyl phosphate as substrate by UV spectroscopy |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13816

([difluoro({4-[3-(4-fluorophenyl)-2-[4-(3-methyl-1,...)Show SMILES Cc1noc(n1)-c1ccc(cc1)C(C\C=C\c1ccccc1)(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)C(=O)c1ccc(F)cc1 Show InChI InChI=1S/C34H28F3N2O5P/c1-23-38-32(44-39-23)27-11-17-28(18-12-27)33(21-5-8-24-6-3-2-4-7-24,31(40)26-13-19-30(35)20-14-26)22-25-9-15-29(16-10-25)34(36,37)45(41,42)43/h2-20H,21-22H2,1H3,(H2,41,42,43)/b8-5+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human TCPTP (1 to 296 residues) catalytic domain expressed in Escherichia coli BL21 cells using fluorescein diphosphate as substrate by... |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50326508

(2,2'-(4,4'-(2-(4-phenoxyphenylcarbamoyl)-1,4-pheny...)Show SMILES OC(=O)C(=O)c1ccc(OCc2ccc(COc3ccc(cc3)C(=O)C(O)=O)c(c2)C(=O)Nc2ccc(Oc3ccccc3)cc2)cc1 Show InChI InChI=1S/C37H27NO10/c39-33(36(42)43)24-8-14-28(15-9-24)46-21-23-6-7-26(22-47-29-16-10-25(11-17-29)34(40)37(44)45)32(20-23)35(41)38-27-12-18-31(19-13-27)48-30-4-2-1-3-5-30/h1-20H,21-22H2,(H,38,41)(H,42,43)(H,44,45) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TCPTP (1 to 341 residues) expressed in Escherichia coli using p-nitrophenyl phosphate as substrate by UV spectroscopy |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13990
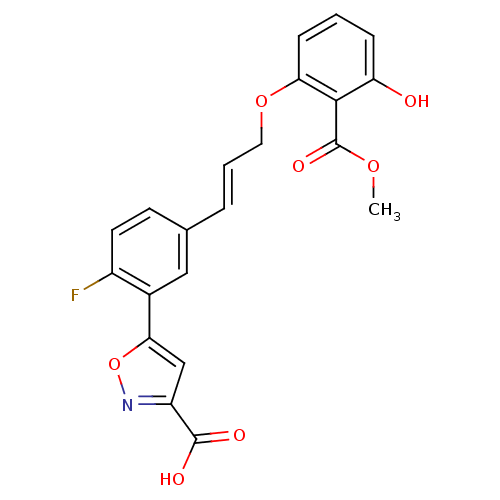
(5-{2-fluoro-5-[(1E)-3-[3-hydroxy-2-(methoxycarbony...)Show SMILES COC(=O)c1c(O)cccc1OC\C=C\c1ccc(F)c(c1)-c1cc(no1)C(O)=O Show InChI InChI=1S/C21H16FNO7/c1-28-21(27)19-16(24)5-2-6-17(19)29-9-3-4-12-7-8-14(22)13(10-12)18-11-15(20(25)26)23-30-18/h2-8,10-11,24H,9H2,1H3,(H,25,26)/b4-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 288 residues) expressed in Escherichia coli BL21 (DE3) cells using p-nitrophenyl phosphate as substrate monitered eve... |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50183056
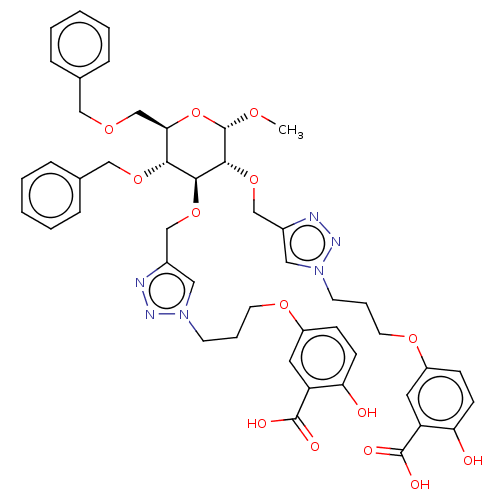
(CHEMBL3818087)Show SMILES CO[C@H]1O[C@H](COCc2ccccc2)[C@@H](OCc2ccccc2)[C@H](OCc2cn(CCCOc3ccc(O)c(c3)C(O)=O)nn2)[C@H]1OCc1cn(CCCOc2ccc(O)c(c2)C(O)=O)nn1 |r| Show InChI InChI=1S/C47H52N6O14/c1-60-47-44(66-29-34-25-53(51-49-34)19-9-21-63-36-15-17-40(55)38(23-36)46(58)59)43(42(64-27-32-12-6-3-7-13-32)41(67-47)30-61-26-31-10-4-2-5-11-31)65-28-33-24-52(50-48-33)18-8-20-62-35-14-16-39(54)37(22-35)45(56)57/h2-7,10-17,22-25,41-44,47,54-55H,8-9,18-21,26-30H2,1H3,(H,56,57)(H,58,59)/t41-,42-,43+,44-,47+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B catalytic domain using p-nitrophenyl phosphate as substrate by micro plate reader method |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50183061
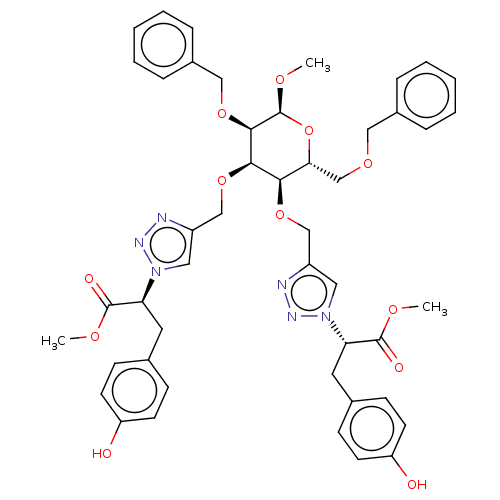
(CHEMBL3818579)Show SMILES CO[C@H]1O[C@H](COCc2ccccc2)[C@@H](OCc2cn(nn2)[C@@H](Cc2ccc(O)cc2)C(=O)OC)[C@@H](OCc2cn(nn2)[C@@H](Cc2ccc(O)cc2)C(=O)OC)[C@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C47H52N6O12/c1-58-45(56)39(22-31-14-18-37(54)19-15-31)52-24-35(48-50-52)28-63-42-41(30-61-26-33-10-6-4-7-11-33)65-47(60-3)44(62-27-34-12-8-5-9-13-34)43(42)64-29-36-25-53(51-49-36)40(46(57)59-2)23-32-16-20-38(55)21-17-32/h4-21,24-25,39-44,47,54-55H,22-23,26-30H2,1-3H3/t39-,40-,41+,42+,43+,44+,47-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B catalytic domain expressed in Escherichia coli BL21 (DE3) cells using p-Nitrophenyl phosphate as substrate by m... |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50183055

(CHEMBL3818449)Show SMILES CO[C@H]1O[C@H](Cn2cc(NC(=O)c3cccc(c3)C(=O)Nc3cn(C[C@H]4O[C@H](OC)[C@H](OCc5ccccc5)[C@H](OCc5ccccc5)[C@@H]4OCc4ccccc4)nn3)nn2)[C@@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C68H70N8O12/c1-79-67-63(85-45-51-30-17-7-18-31-51)61(83-43-49-26-13-5-14-27-49)59(81-41-47-22-9-3-10-23-47)55(87-67)37-75-39-57(71-73-75)69-65(77)53-34-21-35-54(36-53)66(78)70-58-40-76(74-72-58)38-56-60(82-42-48-24-11-4-12-25-48)62(84-44-50-28-15-6-16-29-50)64(68(80-2)88-56)86-46-52-32-19-8-20-33-52/h3-36,39-40,55-56,59-64,67-68H,37-38,41-46H2,1-2H3,(H,69,77)(H,70,78)/t55-,56-,59-,60-,61-,62-,63-,64-,67+,68+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human TCPTP using p-nitrophenyl phosphate as substrate by micro plate reader method |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13997

(5-{3-[(1E)-3-[3-hydroxy-2-(methoxycarbonyl)phenoxy...)Show SMILES COC(=O)c1c(O)cccc1OC\C=C\c1cccc(c1)-c1onc(C(O)=O)c1CO Show InChI InChI=1S/C22H19NO8/c1-29-22(28)18-16(25)8-3-9-17(18)30-10-4-6-13-5-2-7-14(11-13)20-15(12-24)19(21(26)27)23-31-20/h2-9,11,24-25H,10,12H2,1H3,(H,26,27)/b6-4+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP (unknown origin) |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50183056

(CHEMBL3818087)Show SMILES CO[C@H]1O[C@H](COCc2ccccc2)[C@@H](OCc2ccccc2)[C@H](OCc2cn(CCCOc3ccc(O)c(c3)C(O)=O)nn2)[C@H]1OCc1cn(CCCOc2ccc(O)c(c2)C(O)=O)nn1 |r| Show InChI InChI=1S/C47H52N6O14/c1-60-47-44(66-29-34-25-53(51-49-34)19-9-21-63-36-15-17-40(55)38(23-36)46(58)59)43(42(64-27-32-12-6-3-7-13-32)41(67-47)30-61-26-31-10-4-2-5-11-31)65-28-33-24-52(50-48-33)18-8-20-62-35-14-16-39(54)37(22-35)45(56)57/h2-7,10-17,22-25,41-44,47,54-55H,8-9,18-21,26-30H2,1H3,(H,56,57)(H,58,59)/t41-,42-,43+,44-,47+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human TCPTP using p-nitrophenyl phosphate as substrate by micro plate reader method |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50183061

(CHEMBL3818579)Show SMILES CO[C@H]1O[C@H](COCc2ccccc2)[C@@H](OCc2cn(nn2)[C@@H](Cc2ccc(O)cc2)C(=O)OC)[C@@H](OCc2cn(nn2)[C@@H](Cc2ccc(O)cc2)C(=O)OC)[C@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C47H52N6O12/c1-58-45(56)39(22-31-14-18-37(54)19-15-31)52-24-35(48-50-52)28-63-42-41(30-61-26-33-10-6-4-7-11-33)65-47(60-3)44(62-27-34-12-8-5-9-13-34)43(42)64-29-36-25-53(51-49-36)40(46(57)59-2)23-32-16-20-38(55)21-17-32/h4-21,24-25,39-44,47,54-55H,22-23,26-30H2,1-3H3/t39-,40-,41+,42+,43+,44+,47-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human GST-tagged TCPTP expressed in Escherichia coli BL21 (DE3) cells using p-Nitrophenyl phosphate as substrate by microplate reader m... |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13990

(5-{2-fluoro-5-[(1E)-3-[3-hydroxy-2-(methoxycarbony...)Show SMILES COC(=O)c1c(O)cccc1OC\C=C\c1ccc(F)c(c1)-c1cc(no1)C(O)=O Show InChI InChI=1S/C21H16FNO7/c1-28-21(27)19-16(24)5-2-6-17(19)29-9-3-4-12-7-8-14(22)13(10-12)18-11-15(20(25)26)23-30-18/h2-8,10-11,24H,9H2,1H3,(H,25,26)/b4-3+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.64E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP (unknown origin) |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data