 Found 63 hits Enz. Inhib. hit(s) with all data for entry = 50034779
Found 63 hits Enz. Inhib. hit(s) with all data for entry = 50034779 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50283602
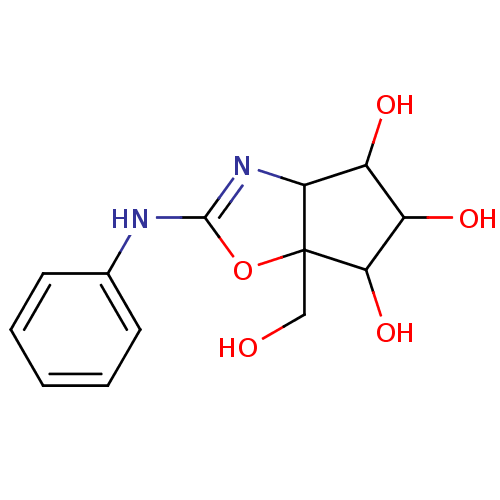
(6a-Hydroxymethyl-2-phenylamino-4,5,6,6a-tetrahydro...)Show InChI InChI=1S/C13H16N2O5/c16-6-13-10(8(17)9(18)11(13)19)15-12(20-13)14-7-4-2-1-3-5-7/h1-5,8-11,16-19H,6H2,(H,14,15) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against baker's yeast Alpha-Glucosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50283601
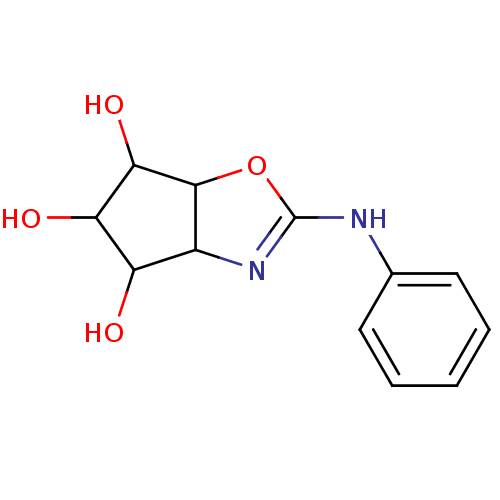
(2-Phenylamino-4,5,6,6a-tetrahydro-3aH-cyclopentaox...)Show InChI InChI=1S/C12H14N2O4/c15-8-7-11(10(17)9(8)16)18-12(14-7)13-6-4-2-1-3-5-6/h1-5,7-11,15-17H,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Escherichia coli beta galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50283601

(2-Phenylamino-4,5,6,6a-tetrahydro-3aH-cyclopentaox...)Show InChI InChI=1S/C12H14N2O4/c15-8-7-11(10(17)9(8)16)18-12(14-7)13-6-4-2-1-3-5-6/h1-5,7-11,15-17H,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Escherichia coli beta galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Bos taurus) | BDBM50283601

(2-Phenylamino-4,5,6,6a-tetrahydro-3aH-cyclopentaox...)Show InChI InChI=1S/C12H14N2O4/c15-8-7-11(10(17)9(8)16)18-12(14-7)13-6-4-2-1-3-5-6/h1-5,7-11,15-17H,(H,13,14) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Escherichia coli beta galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50283602

(6a-Hydroxymethyl-2-phenylamino-4,5,6,6a-tetrahydro...)Show InChI InChI=1S/C13H16N2O5/c16-6-13-10(8(17)9(18)11(13)19)15-12(20-13)14-7-4-2-1-3-5-7/h1-5,8-11,16-19H,6H2,(H,14,15) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >999 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against baker's yeast Alpha-Glucosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50283602

(6a-Hydroxymethyl-2-phenylamino-4,5,6,6a-tetrahydro...)Show InChI InChI=1S/C13H16N2O5/c16-6-13-10(8(17)9(18)11(13)19)15-12(20-13)14-7-4-2-1-3-5-7/h1-5,8-11,16-19H,6H2,(H,14,15) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >999 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against baker's yeast Alpha-Glucosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50283598
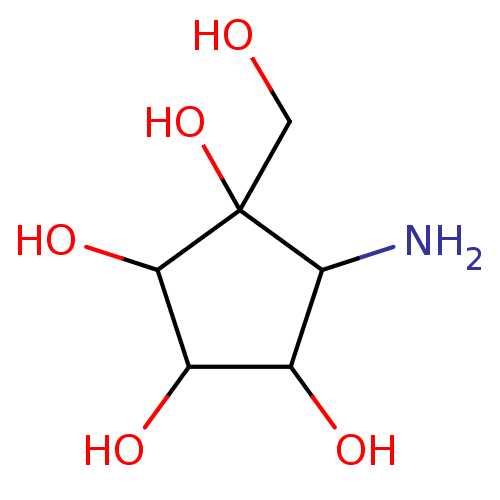
(5-Amino-1-hydroxymethyl-cyclopentane-1,2,3,4-tetra...)Show InChI InChI=1S/C6H13NO5/c7-4-2(9)3(10)5(11)6(4,12)1-8/h2-5,8-12H,1,7H2 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against baker's yeast Alpha-galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Bos taurus) | BDBM50283602

(6a-Hydroxymethyl-2-phenylamino-4,5,6,6a-tetrahydro...)Show InChI InChI=1S/C13H16N2O5/c16-6-13-10(8(17)9(18)11(13)19)15-12(20-13)14-7-4-2-1-3-5-7/h1-5,8-11,16-19H,6H2,(H,14,15) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against bovine liver beta galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50283601

(2-Phenylamino-4,5,6,6a-tetrahydro-3aH-cyclopentaox...)Show InChI InChI=1S/C12H14N2O4/c15-8-7-11(10(17)9(8)16)18-12(14-7)13-6-4-2-1-3-5-6/h1-5,7-11,15-17H,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against almonds Beta-glucosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50283601

(2-Phenylamino-4,5,6,6a-tetrahydro-3aH-cyclopentaox...)Show InChI InChI=1S/C12H14N2O4/c15-8-7-11(10(17)9(8)16)18-12(14-7)13-6-4-2-1-3-5-6/h1-5,7-11,15-17H,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against almonds Beta-glucosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50283603
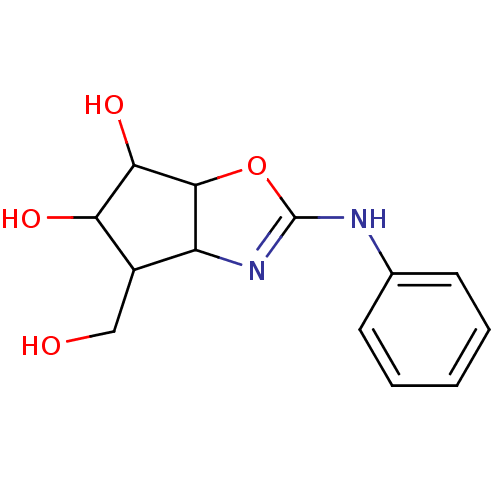
(4-Hydroxymethyl-2-phenylamino-4,5,6,6a-tetrahydro-...)Show InChI InChI=1S/C13H16N2O4/c16-6-8-9-12(11(18)10(8)17)19-13(15-9)14-7-4-2-1-3-5-7/h1-5,8-12,16-18H,6H2,(H,14,15) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against baker's yeast Alpha-Glucosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50283604
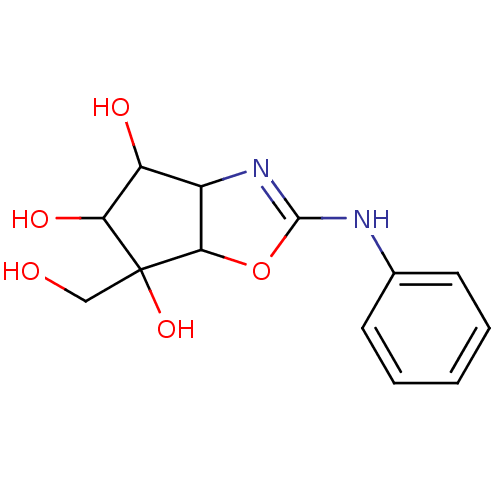
(6-Hydroxymethyl-2-phenylamino-4,5,6,6a-tetrahydro-...)Show InChI InChI=1S/C13H16N2O5/c16-6-13(19)10(18)9(17)8-11(13)20-12(15-8)14-7-4-2-1-3-5-7/h1-5,8-11,16-19H,6H2,(H,14,15) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against baker's yeast Alpha-Glucosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Bos taurus) | BDBM50283603

(4-Hydroxymethyl-2-phenylamino-4,5,6,6a-tetrahydro-...)Show InChI InChI=1S/C13H16N2O4/c16-6-8-9-12(11(18)10(8)17)19-13(15-9)14-7-4-2-1-3-5-7/h1-5,8-12,16-18H,6H2,(H,14,15) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against bovine liver beta galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50283602

(6a-Hydroxymethyl-2-phenylamino-4,5,6,6a-tetrahydro...)Show InChI InChI=1S/C13H16N2O5/c16-6-13-10(8(17)9(18)11(13)19)15-12(20-13)14-7-4-2-1-3-5-7/h1-5,8-11,16-19H,6H2,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Escherichia coli Alpha-galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50283601

(2-Phenylamino-4,5,6,6a-tetrahydro-3aH-cyclopentaox...)Show InChI InChI=1S/C12H14N2O4/c15-8-7-11(10(17)9(8)16)18-12(14-7)13-6-4-2-1-3-5-6/h1-5,7-11,15-17H,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against almonds Beta-glucosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50283600
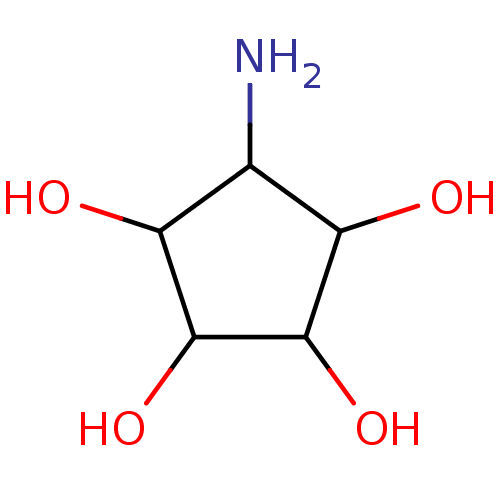
(5-Amino-cyclopentane-1,2,3,4-tetraol | CHEMBL87120)Show InChI InChI=1S/C5H11NO4/c6-1-2(7)4(9)5(10)3(1)8/h1-5,7-10H,6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
| n/a | n/a | 7.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Escherichia coli beta galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50283600

(5-Amino-cyclopentane-1,2,3,4-tetraol | CHEMBL87120)Show InChI InChI=1S/C5H11NO4/c6-1-2(7)4(9)5(10)3(1)8/h1-5,7-10H,6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
| n/a | n/a | 7.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Escherichia coli beta galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Bos taurus) | BDBM50283599
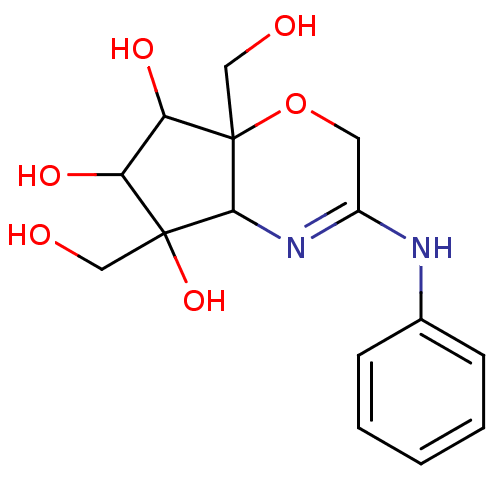
(5,7a-Bis-hydroxymethyl-3-phenylamino-2,4a,5,6,7,7a...)Show SMILES OCC1(O)C(O)C(O)C2(CO)OCC(Nc3ccccc3)=NC12 |c:21| Show InChI InChI=1S/C15H20N2O6/c18-7-14(22)11(20)12(21)15(8-19)13(14)17-10(6-23-15)16-9-4-2-1-3-5-9/h1-5,11-13,18-22H,6-8H2,(H,16,17) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 2.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against bovine liver beta galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50283598

(5-Amino-1-hydroxymethyl-cyclopentane-1,2,3,4-tetra...)Show InChI InChI=1S/C6H13NO5/c7-4-2(9)3(10)5(11)6(4,12)1-8/h2-5,8-12H,1,7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against almonds beta-glucosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Bos taurus) | BDBM50283600

(5-Amino-cyclopentane-1,2,3,4-tetraol | CHEMBL87120)Show InChI InChI=1S/C5H11NO4/c6-1-2(7)4(9)5(10)3(1)8/h1-5,7-10H,6H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
| n/a | n/a | 4.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against bovine liver beta galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Bos taurus) | BDBM50283600

(5-Amino-cyclopentane-1,2,3,4-tetraol | CHEMBL87120)Show InChI InChI=1S/C5H11NO4/c6-1-2(7)4(9)5(10)3(1)8/h1-5,7-10H,6H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
| n/a | n/a | 4.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against bovine liver beta galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Bos taurus) | BDBM50283602

(6a-Hydroxymethyl-2-phenylamino-4,5,6,6a-tetrahydro...)Show InChI InChI=1S/C13H16N2O5/c16-6-13-10(8(17)9(18)11(13)19)15-12(20-13)14-7-4-2-1-3-5-7/h1-5,8-11,16-19H,6H2,(H,14,15) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against bovine liver beta galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Bos taurus) | BDBM50283602

(6a-Hydroxymethyl-2-phenylamino-4,5,6,6a-tetrahydro...)Show InChI InChI=1S/C13H16N2O5/c16-6-13-10(8(17)9(18)11(13)19)15-12(20-13)14-7-4-2-1-3-5-7/h1-5,8-11,16-19H,6H2,(H,14,15) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against bovine liver beta galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50283600

(5-Amino-cyclopentane-1,2,3,4-tetraol | CHEMBL87120)Show InChI InChI=1S/C5H11NO4/c6-1-2(7)4(9)5(10)3(1)8/h1-5,7-10H,6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
| n/a | n/a | 8.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against almonds beta-glucosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50283600

(5-Amino-cyclopentane-1,2,3,4-tetraol | CHEMBL87120)Show InChI InChI=1S/C5H11NO4/c6-1-2(7)4(9)5(10)3(1)8/h1-5,7-10H,6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
| n/a | n/a | 8.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against almonds beta-glucosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM18351

((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5-,6-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 9.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against baker's yeast Alpha-Glucosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50283599

(5,7a-Bis-hydroxymethyl-3-phenylamino-2,4a,5,6,7,7a...)Show SMILES OCC1(O)C(O)C(O)C2(CO)OCC(Nc3ccccc3)=NC12 |c:21| Show InChI InChI=1S/C15H20N2O6/c18-7-14(22)11(20)12(21)15(8-19)13(14)17-10(6-23-15)16-9-4-2-1-3-5-9/h1-5,11-13,18-22H,6-8H2,(H,16,17) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Escherichia coli Alpha-galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM18351

((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5-,6-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Escherichia coli Alpha-galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Bos taurus) | BDBM50283604

(6-Hydroxymethyl-2-phenylamino-4,5,6,6a-tetrahydro-...)Show InChI InChI=1S/C13H16N2O5/c16-6-13(19)10(18)9(17)8-11(13)20-12(15-8)14-7-4-2-1-3-5-7/h1-5,8-11,16-19H,6H2,(H,14,15) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.53E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against bovine liver beta galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50283598

(5-Amino-1-hydroxymethyl-cyclopentane-1,2,3,4-tetra...)Show InChI InChI=1S/C6H13NO5/c7-4-2(9)3(10)5(11)6(4,12)1-8/h2-5,8-12H,1,7H2 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.62E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against baker's yeast Alpha-Glucosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50283598

(5-Amino-1-hydroxymethyl-cyclopentane-1,2,3,4-tetra...)Show InChI InChI=1S/C6H13NO5/c7-4-2(9)3(10)5(11)6(4,12)1-8/h2-5,8-12H,1,7H2 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.62E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against baker's yeast Alpha-Glucosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50283599

(5,7a-Bis-hydroxymethyl-3-phenylamino-2,4a,5,6,7,7a...)Show SMILES OCC1(O)C(O)C(O)C2(CO)OCC(Nc3ccccc3)=NC12 |c:21| Show InChI InChI=1S/C15H20N2O6/c18-7-14(22)11(20)12(21)15(8-19)13(14)17-10(6-23-15)16-9-4-2-1-3-5-9/h1-5,11-13,18-22H,6-8H2,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 2.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against almonds Beta-glucosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50283601

(2-Phenylamino-4,5,6,6a-tetrahydro-3aH-cyclopentaox...)Show InChI InChI=1S/C12H14N2O4/c15-8-7-11(10(17)9(8)16)18-12(14-7)13-6-4-2-1-3-5-6/h1-5,7-11,15-17H,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Escherichia coli beta galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50283603

(4-Hydroxymethyl-2-phenylamino-4,5,6,6a-tetrahydro-...)Show InChI InChI=1S/C13H16N2O4/c16-6-8-9-12(11(18)10(8)17)19-13(15-9)14-7-4-2-1-3-5-7/h1-5,8-12,16-18H,6H2,(H,14,15) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Escherichia coli Alpha-galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50283600

(5-Amino-cyclopentane-1,2,3,4-tetraol | CHEMBL87120)Show InChI InChI=1S/C5H11NO4/c6-1-2(7)4(9)5(10)3(1)8/h1-5,7-10H,6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against almonds beta-glucosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50283600

(5-Amino-cyclopentane-1,2,3,4-tetraol | CHEMBL87120)Show InChI InChI=1S/C5H11NO4/c6-1-2(7)4(9)5(10)3(1)8/h1-5,7-10H,6H2 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against baker's yeast Alpha-Glucosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50283598

(5-Amino-1-hydroxymethyl-cyclopentane-1,2,3,4-tetra...)Show InChI InChI=1S/C6H13NO5/c7-4-2(9)3(10)5(11)6(4,12)1-8/h2-5,8-12H,1,7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Escherichia coli beta galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50283602

(6a-Hydroxymethyl-2-phenylamino-4,5,6,6a-tetrahydro...)Show InChI InChI=1S/C13H16N2O5/c16-6-13-10(8(17)9(18)11(13)19)15-12(20-13)14-7-4-2-1-3-5-7/h1-5,8-11,16-19H,6H2,(H,14,15) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Escherichia coli Alpha-galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50283598

(5-Amino-1-hydroxymethyl-cyclopentane-1,2,3,4-tetra...)Show InChI InChI=1S/C6H13NO5/c7-4-2(9)3(10)5(11)6(4,12)1-8/h2-5,8-12H,1,7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against almonds beta-glucosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50283600

(5-Amino-cyclopentane-1,2,3,4-tetraol | CHEMBL87120)Show InChI InChI=1S/C5H11NO4/c6-1-2(7)4(9)5(10)3(1)8/h1-5,7-10H,6H2 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Escherichia coli Alpha-galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50283600

(5-Amino-cyclopentane-1,2,3,4-tetraol | CHEMBL87120)Show InChI InChI=1S/C5H11NO4/c6-1-2(7)4(9)5(10)3(1)8/h1-5,7-10H,6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against almonds beta-glucosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Bos taurus) | BDBM50283600

(5-Amino-cyclopentane-1,2,3,4-tetraol | CHEMBL87120)Show InChI InChI=1S/C5H11NO4/c6-1-2(7)4(9)5(10)3(1)8/h1-5,7-10H,6H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Escherichia coli Alpha-galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50283602

(6a-Hydroxymethyl-2-phenylamino-4,5,6,6a-tetrahydro...)Show InChI InChI=1S/C13H16N2O5/c16-6-13-10(8(17)9(18)11(13)19)15-12(20-13)14-7-4-2-1-3-5-7/h1-5,8-11,16-19H,6H2,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against almonds Beta-glucosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50283598

(5-Amino-1-hydroxymethyl-cyclopentane-1,2,3,4-tetra...)Show InChI InChI=1S/C6H13NO5/c7-4-2(9)3(10)5(11)6(4,12)1-8/h2-5,8-12H,1,7H2 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Escherichia coli beta galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Bos taurus) | BDBM50283598

(5-Amino-1-hydroxymethyl-cyclopentane-1,2,3,4-tetra...)Show InChI InChI=1S/C6H13NO5/c7-4-2(9)3(10)5(11)6(4,12)1-8/h2-5,8-12H,1,7H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Escherichia coli Alpha-galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50283599

(5,7a-Bis-hydroxymethyl-3-phenylamino-2,4a,5,6,7,7a...)Show SMILES OCC1(O)C(O)C(O)C2(CO)OCC(Nc3ccccc3)=NC12 |c:21| Show InChI InChI=1S/C15H20N2O6/c18-7-14(22)11(20)12(21)15(8-19)13(14)17-10(6-23-15)16-9-4-2-1-3-5-9/h1-5,11-13,18-22H,6-8H2,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Escherichia coli beta galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50283604

(6-Hydroxymethyl-2-phenylamino-4,5,6,6a-tetrahydro-...)Show InChI InChI=1S/C13H16N2O5/c16-6-13(19)10(18)9(17)8-11(13)20-12(15-8)14-7-4-2-1-3-5-7/h1-5,8-11,16-19H,6H2,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against baker's yeast Alpha-Glucosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Bos taurus) | BDBM50283601

(2-Phenylamino-4,5,6,6a-tetrahydro-3aH-cyclopentaox...)Show InChI InChI=1S/C12H14N2O4/c15-8-7-11(10(17)9(8)16)18-12(14-7)13-6-4-2-1-3-5-6/h1-5,7-11,15-17H,(H,13,14) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against baker's yeast Alpha-Glucosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50283603

(4-Hydroxymethyl-2-phenylamino-4,5,6,6a-tetrahydro-...)Show InChI InChI=1S/C13H16N2O4/c16-6-8-9-12(11(18)10(8)17)19-13(15-9)14-7-4-2-1-3-5-7/h1-5,8-12,16-18H,6H2,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Escherichia coli Alpha-galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50283601

(2-Phenylamino-4,5,6,6a-tetrahydro-3aH-cyclopentaox...)Show InChI InChI=1S/C12H14N2O4/c15-8-7-11(10(17)9(8)16)18-12(14-7)13-6-4-2-1-3-5-6/h1-5,7-11,15-17H,(H,13,14) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against Escherichia coli beta galactosidase |
Bioorg Med Chem Lett 4: 2643-2648 (1994)
Article DOI: 10.1016/S0960-894X(01)80688-0
BindingDB Entry DOI: 10.7270/Q21V5FFN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data