

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM25164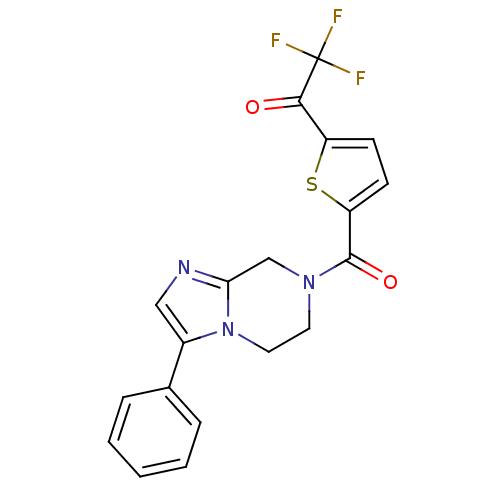 (2,2,2-trifluoro-1-[5-({3-phenyl-5H,6H,7H,8H-imidaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 367 | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University Curated by ChEMBL | Assay Description Inhibition of human HDAC4 catalytic domain (residues Thr648-Thr1057) expressed in Escherichia coli BL21using fluorogenic substrate by fluorescence as... | Bioorg Med Chem Lett 21: 5854-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.100 BindingDB Entry DOI: 10.7270/Q2KS6RZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50354831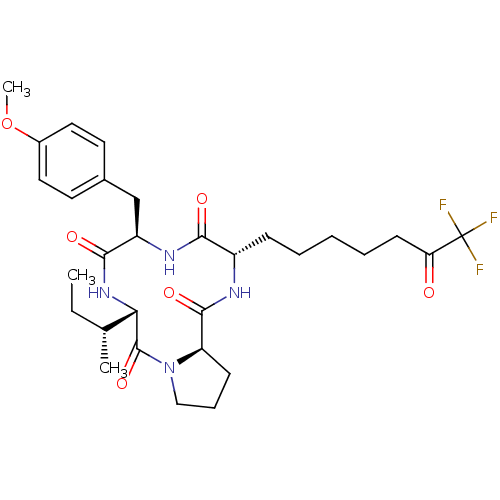 (CHEMBL1833976) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University Curated by ChEMBL | Assay Description Inhibition of HDAC8 | Bioorg Med Chem Lett 21: 5854-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.100 BindingDB Entry DOI: 10.7270/Q2KS6RZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50121062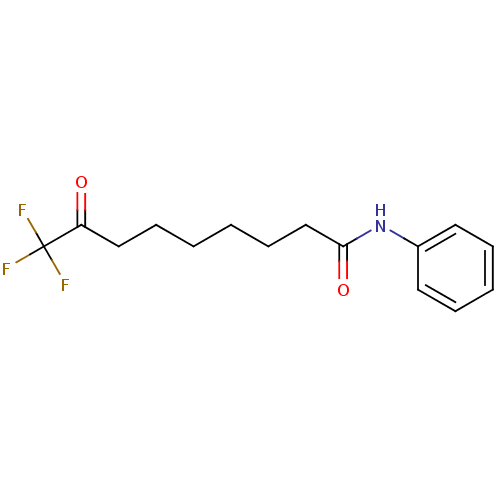 (9,9,9-TRIFLUORO-8-OXO-N-PHENYLNONANAMIDE | 9,9,9-T...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University Curated by ChEMBL | Assay Description Inhibition of HDAC8 assessed as substrate deacetylation using N-acetyl-L-Arg-LHis-L-Lys(epsilon-acetyl)-L-Lys(epsilon-acetyl)-coumarin as substrate p... | Bioorg Med Chem Lett 21: 5854-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.100 BindingDB Entry DOI: 10.7270/Q2KS6RZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylpolyamine amidohydrolase (Mycoplana ramosa (Gram-negative bacterium)) | BDBM50121062 (9,9,9-TRIFLUORO-8-OXO-N-PHENYLNONANAMIDE | 9,9,9-T...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University Curated by ChEMBL | Assay Description Inhibition of Mycoplana ramosa acetylpolyamine amidohydrolase assessed as substrate deacetylation using L-Lys(epsilon-acetyl)-coumarin as substrate p... | Bioorg Med Chem Lett 21: 5854-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.100 BindingDB Entry DOI: 10.7270/Q2KS6RZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50354832 (CHEMBL1834160) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a |
Drexel University Curated by ChEMBL | Assay Description Displacement of [14C-guanidino]-L-arginine from human recombinant full length arginase 1 expressed in Escherichia coli BL21(DE3) by fixed point assay | Bioorg Med Chem Lett 21: 5854-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.100 BindingDB Entry DOI: 10.7270/Q2KS6RZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||