

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50254782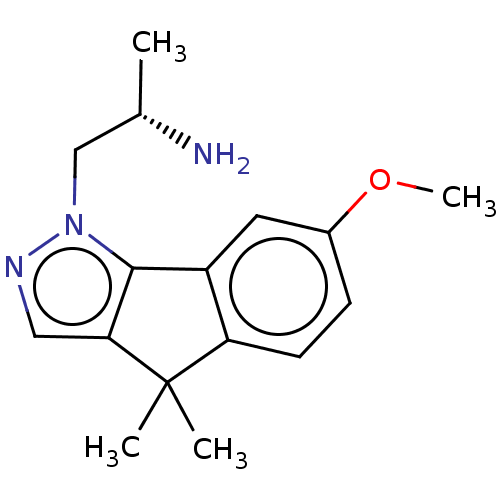 (CHEMBL4086240) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Agonist activity at human 5-HT2C receptor expressed in mouse NIH/3T3 cells assessed as induction of IP3 formation after 0.5 hrs | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50254783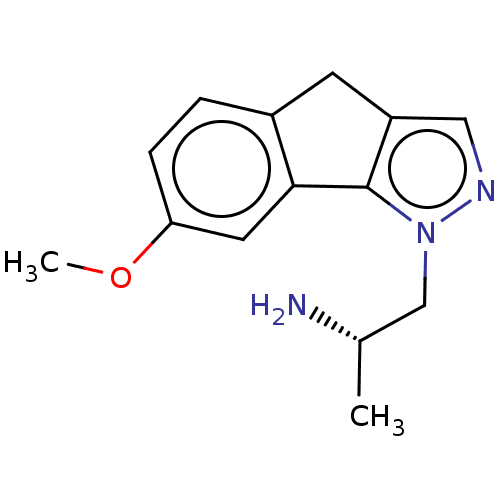 (CHEMBL4092042) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Agonist activity at human 5-HT2C receptor expressed in mouse NIH/3T3 cells assessed as induction of IP3 formation after 0.5 hrs | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001859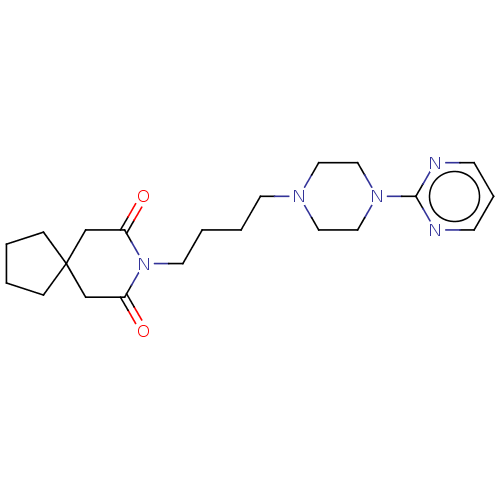 ((buspirone) 8-[4-(4-Pyrimidin-2-yl-piperazin-1-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Binding affinity to 5-HT1A (unknown origin) | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50038001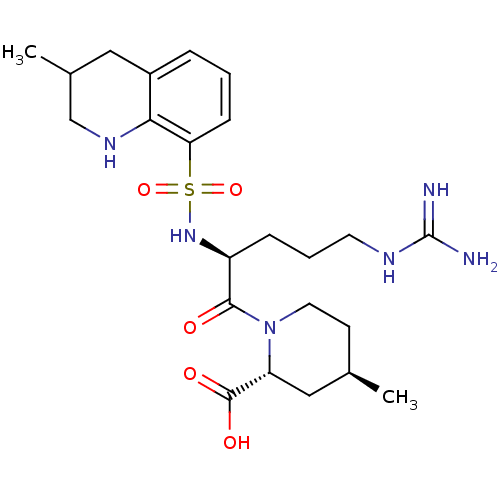 ((2R,4R)-1-((S)-5-(diaminomethyleneamino)-2-(3-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of bovine plasma thrombin using chromogenix AB as substrate after 30 secs by UV-spectrophotometry | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50254781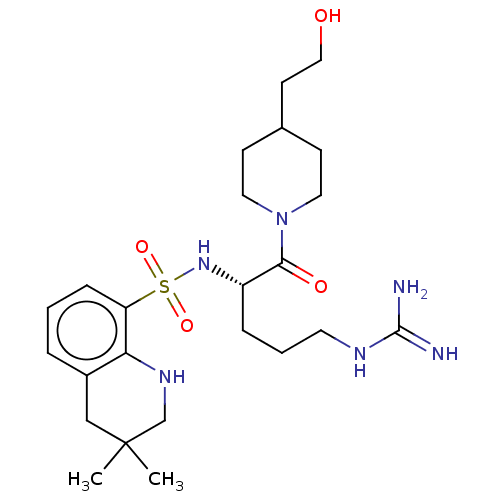 (CHEMBL4083414) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of human plasma thrombin using chromogenix AB as substrate after 30 secs by UV-spectrophotometry | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50005132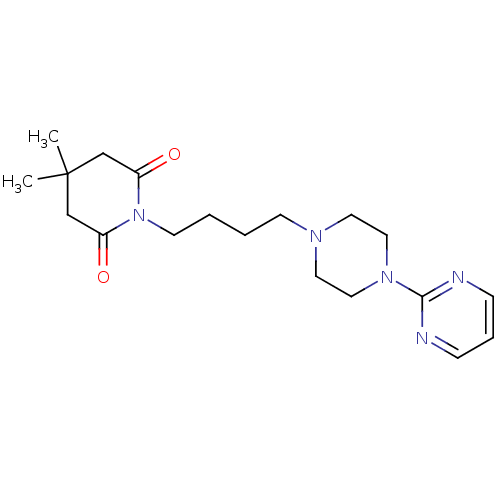 (4,4-Dimethyl-1-[4-(4-pyrimidin-2-yl-piperazin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Binding affinity to 5-HT1A (unknown origin) | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50038001 ((2R,4R)-1-((S)-5-(diaminomethyleneamino)-2-(3-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of human plasma thrombin using chromogenix AB as substrate after 30 secs by UV-spectrophotometry | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50254811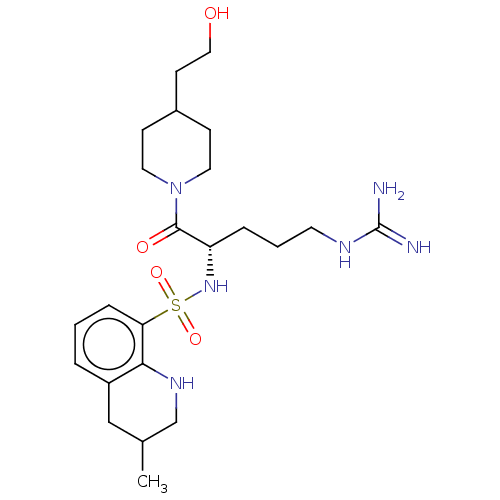 (CHEMBL4091198) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of human plasma thrombin using chromogenix AB as substrate after 30 secs by UV-spectrophotometry | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50254781 (CHEMBL4083414) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of bovine plasma thrombin using chromogenix AB as substrate after 30 secs by UV-spectrophotometry | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50254811 (CHEMBL4091198) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of bovine plasma thrombin using chromogenix AB as substrate after 30 secs by UV-spectrophotometry | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50254803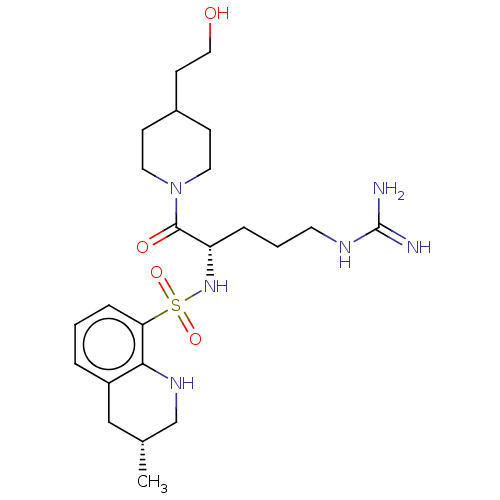 (CHEMBL4101401) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of bovine plasma thrombin using chromogenix AB as substrate after 30 secs by UV-spectrophotometry | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001859 ((buspirone) 8-[4-(4-Pyrimidin-2-yl-piperazin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 484 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Displacement of [3H]methylspiperone from human D2 receptor expressed in HEK cells | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50254804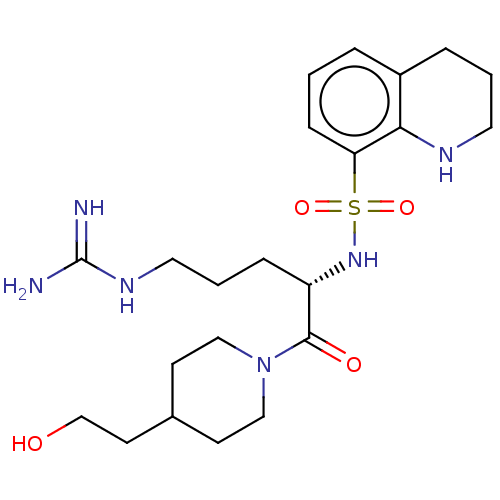 (CHEMBL4063331) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of bovine plasma thrombin using chromogenix AB as substrate after 30 secs by UV-spectrophotometry | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50001859 ((buspirone) 8-[4-(4-Pyrimidin-2-yl-piperazin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Binding affinity to 5-HT2A (unknown origin) | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50005132 (4,4-Dimethyl-1-[4-(4-pyrimidin-2-yl-piperazin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Binding affinity to 5-HT2A (unknown origin) | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50005132 (4,4-Dimethyl-1-[4-(4-pyrimidin-2-yl-piperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Displacement of [3H]methylspiperone from human D2 receptor expressed in HEK cells | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50254802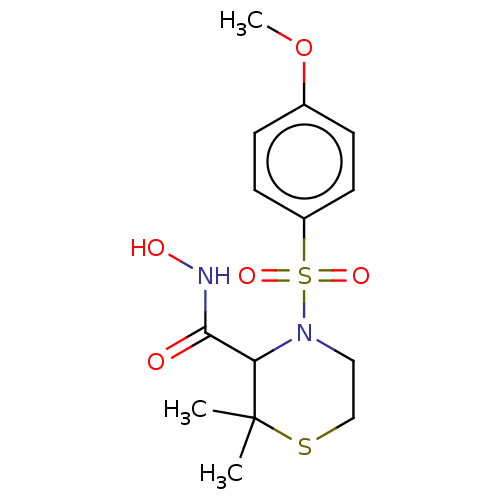 (CHEMBL4088630) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of human interstitial recombinant N-terminal MMP1 expressed in Escherichia coli BL21(DE3) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as sub... | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50254802 (CHEMBL4088630) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of truncated human recombinant MMP3 catalytic domain expressed in Escherichia coli BL21(DE3) after 3 hrs in presence of [H]-transferrin | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50082545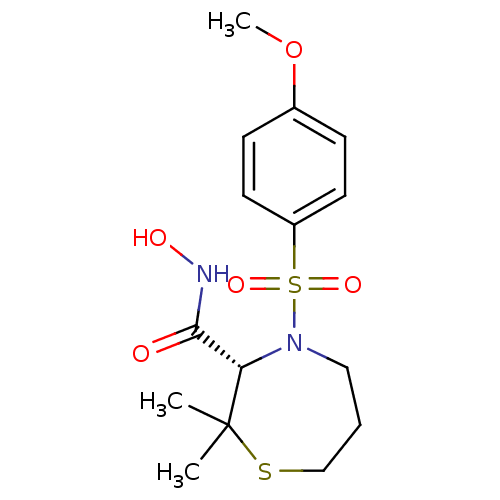 ((S)-4-(4-Methoxy-benzenesulfonyl)-2,2-dimethyl-[1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of truncated human recombinant MMP3 catalytic domain expressed in Escherichia coli BL21(DE3) after 3 hrs in presence of [H]-transferrin | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50254802 (CHEMBL4088630) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of MMP8 (unknown origin) expressed in Escherichia coli BL21(DE3) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate after 20 to 30 min... | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50082545 ((S)-4-(4-Methoxy-benzenesulfonyl)-2,2-dimethyl-[1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of human interstitial recombinant N-terminal MMP1 expressed in Escherichia coli BL21(DE3) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as sub... | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50139181 ((1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotet...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase (unknown origin) using [14C]-HMG-CoA as substrate after 5 mins in presence of NADPH | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50082545 ((S)-4-(4-Methoxy-benzenesulfonyl)-2,2-dimethyl-[1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of MMP13 (unknown origin) expressed in Escherichia coli BL21(DE3) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate after 20 to 30 mi... | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50254802 (CHEMBL4088630) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of MMP9 (unknown origin) expressed in Escherichia coli BL21(DE3) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate after 20 to 30 min... | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50089619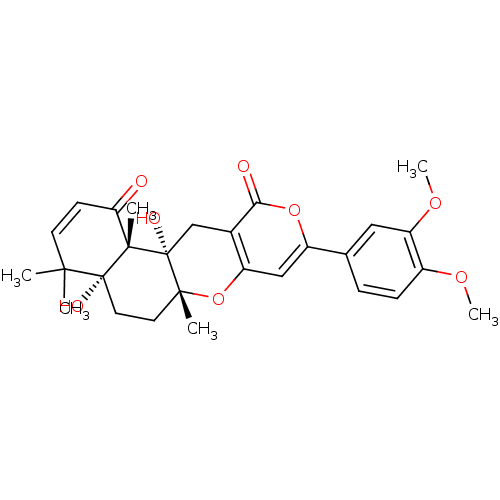 (9-(3,4-Dimethoxy-phenyl)-4a,12a-dihydroxy-4,4,6a,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50082555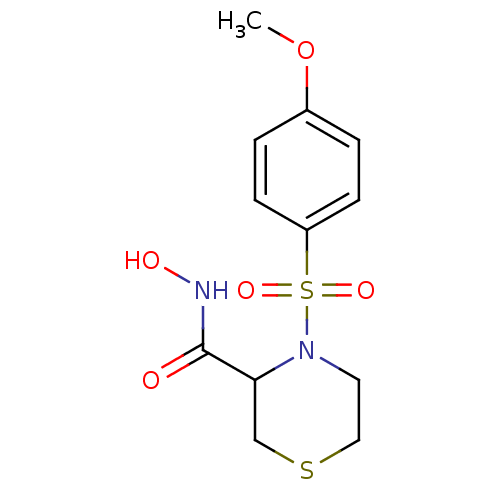 (4-(4-Methoxy-benzenesulfonyl)-thiomorpholine-3-car...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of MMP13 (unknown origin) expressed in Escherichia coli BL21(DE3) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate after 20 to 30 mi... | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50254802 (CHEMBL4088630) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of MMP13 (unknown origin) expressed in Escherichia coli BL21(DE3) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate after 20 to 30 mi... | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50082545 ((S)-4-(4-Methoxy-benzenesulfonyl)-2,2-dimethyl-[1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of MMP8 (unknown origin) expressed in Escherichia coli BL21(DE3) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate after 20 to 30 min... | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50254802 (CHEMBL4088630) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of MMP2 (unknown origin) expressed in Escherichia coli BL21(DE3) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate after 20 to 30 min... | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50082545 ((S)-4-(4-Methoxy-benzenesulfonyl)-2,2-dimethyl-[1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of MMP9 (unknown origin) expressed in Escherichia coli BL21(DE3) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate after 20 to 30 min... | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50254789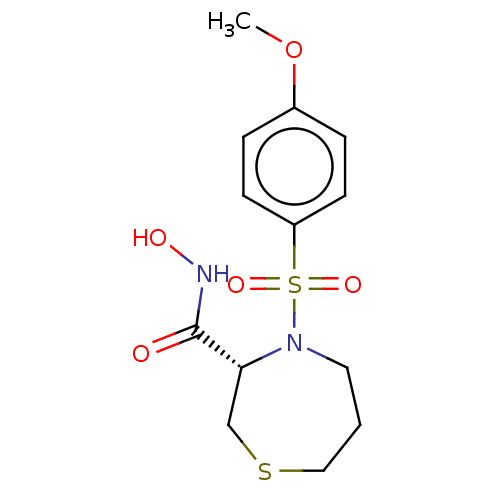 (CHEMBL4067375) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of MMP13 (unknown origin) expressed in Escherichia coli BL21(DE3) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate after 20 to 30 mi... | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50082538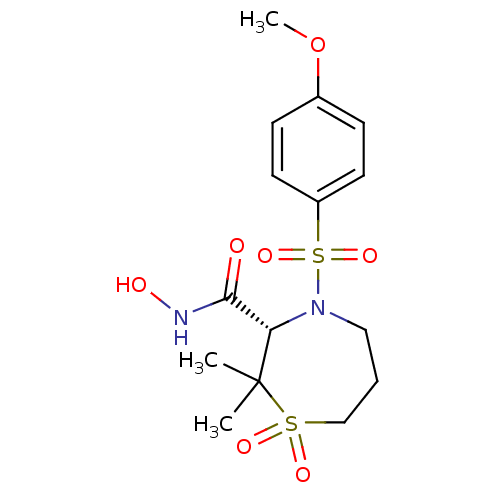 ((S)-4-(4-Methoxy-benzenesulfonyl)-2,2-dimethyl-1,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of human interstitial recombinant N-terminal MMP1 expressed in Escherichia coli BL21(DE3) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as sub... | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50082538 ((S)-4-(4-Methoxy-benzenesulfonyl)-2,2-dimethyl-1,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of MMP13 (unknown origin) expressed in Escherichia coli BL21(DE3) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate after 20 to 30 mi... | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM34168 (LOVASTATIN | MLS000069585 | SMR000058779 | US91151...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase (unknown origin) using [14C]-HMG-CoA as substrate after 5 mins in presence of NADPH | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50254788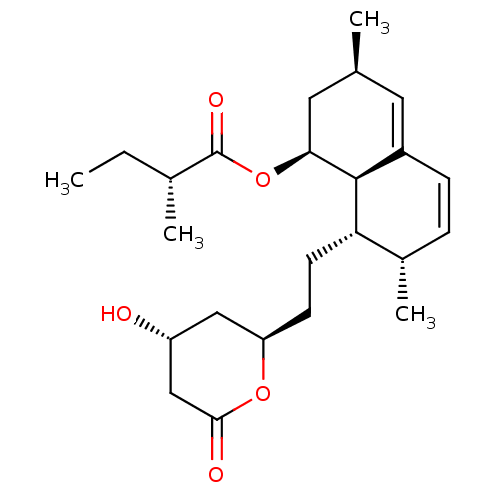 (CHEMBL4088701) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase (unknown origin) using [14C]-HMG-CoA as substrate after 5 mins in presence of NADPH | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50254786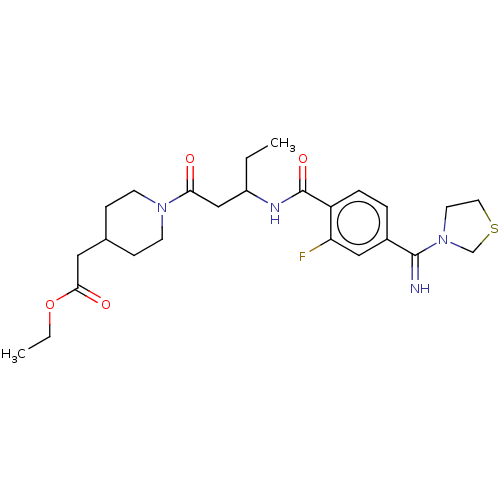 (CHEMBL4102322) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of human GP2b/3a interaction with human fibrinogen after 3 hrs by ELISA | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50082538 ((S)-4-(4-Methoxy-benzenesulfonyl)-2,2-dimethyl-1,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of MMP8 (unknown origin) expressed in Escherichia coli BL21(DE3) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate after 20 to 30 min... | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50066659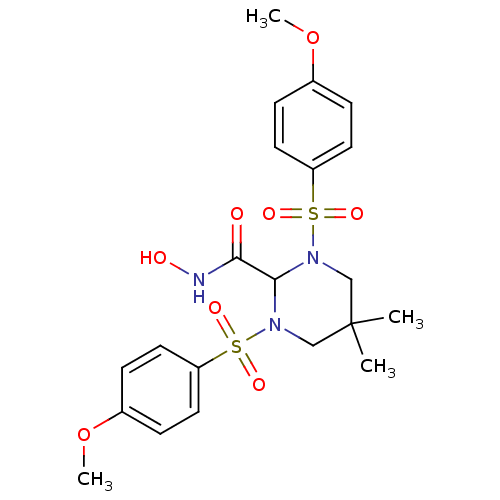 (1,3-BIS-(4-METHOXY-BENZENESULFONYL)-5,5-DIMETHYL-H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of MMP9 (unknown origin) expressed in Escherichia coli BL21(DE3) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate after 20 to 30 min... | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50082545 ((S)-4-(4-Methoxy-benzenesulfonyl)-2,2-dimethyl-[1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of MMP2 (unknown origin) expressed in Escherichia coli BL21(DE3) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate after 20 to 30 min... | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50082538 ((S)-4-(4-Methoxy-benzenesulfonyl)-2,2-dimethyl-1,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of MMP9 (unknown origin) expressed in Escherichia coli BL21(DE3) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate after 20 to 30 min... | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50066657 (1,3-Bis-(4-methoxy-benzenesulfonyl)-hexahydro-pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of MMP9 (unknown origin) expressed in Escherichia coli BL21(DE3) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate after 20 to 30 min... | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50254789 (CHEMBL4067375) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of truncated human recombinant MMP3 catalytic domain expressed in Escherichia coli BL21(DE3) after 3 hrs in presence of [H]-transferrin | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50082538 ((S)-4-(4-Methoxy-benzenesulfonyl)-2,2-dimethyl-1,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of truncated human recombinant MMP3 catalytic domain expressed in Escherichia coli BL21(DE3) after 3 hrs in presence of [H]-transferrin | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50082538 ((S)-4-(4-Methoxy-benzenesulfonyl)-2,2-dimethyl-1,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of MMP2 (unknown origin) expressed in Escherichia coli BL21(DE3) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate after 20 to 30 min... | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50254813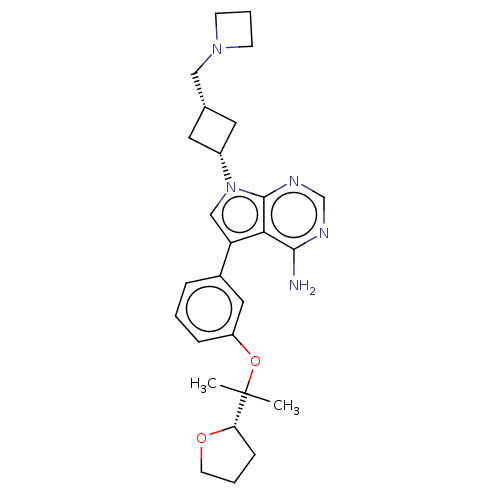 (CHEMBL4096340) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Antagonist activity at human GST-tagged IGF-1R (950 to 133 residues) expressed in baculovirus after 5 mins in presence of [gamma33P]-ATP by top count... | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Homo sapiens (Human)) | BDBM50104844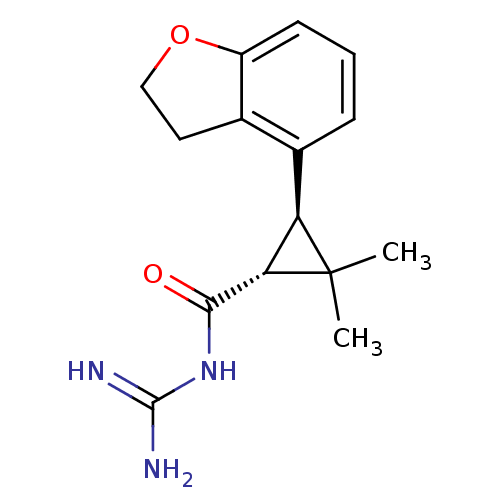 (BMS-284640 | CHEMBL51879 | N-[(1R,3R)-3-(2,3-Dihyd...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of human NHE1 by cell based assay | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50082555 (4-(4-Methoxy-benzenesulfonyl)-thiomorpholine-3-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of truncated human recombinant MMP3 catalytic domain expressed in Escherichia coli BL21(DE3) after 3 hrs in presence of [H]-transferrin | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50066659 (1,3-BIS-(4-METHOXY-BENZENESULFONYL)-5,5-DIMETHYL-H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of truncated human recombinant MMP3 catalytic domain expressed in Escherichia coli BL21(DE3) after 3 hrs in presence of [H]-transferrin | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50092119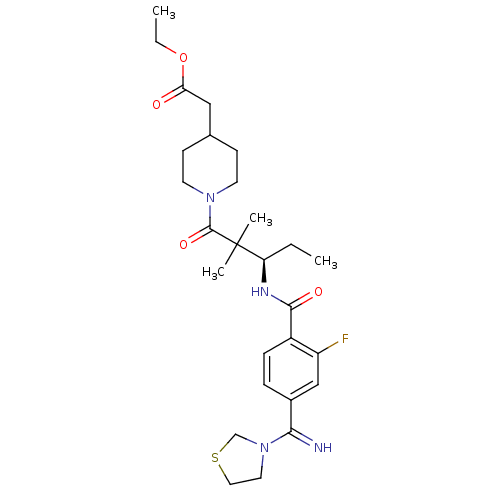 ((1-{3-[2-Fluoro-4-(imino-thiazolidin-3-yl-methyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of GP2b/3a in human platelet-rich plasma assessed as reduction in collagen-induced platelet aggregation preincubated for 1 min followed by... | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50254802 (CHEMBL4088630) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of MMP7 (unknown origin) expressed in Escherichia coli BL21(DE3) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate after 20 to 30 min... | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 85 total ) | Next | Last >> |