

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50258600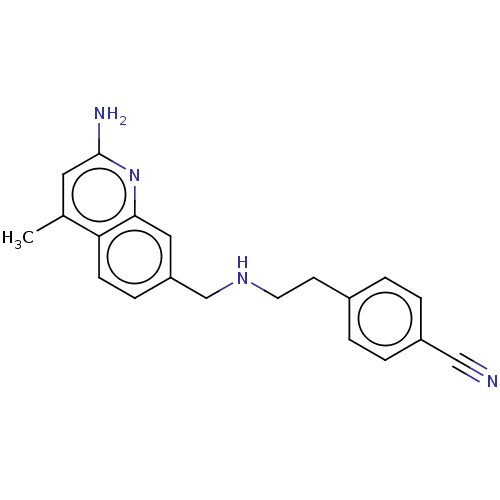 (CHEMBL4064297) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant rat nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50258602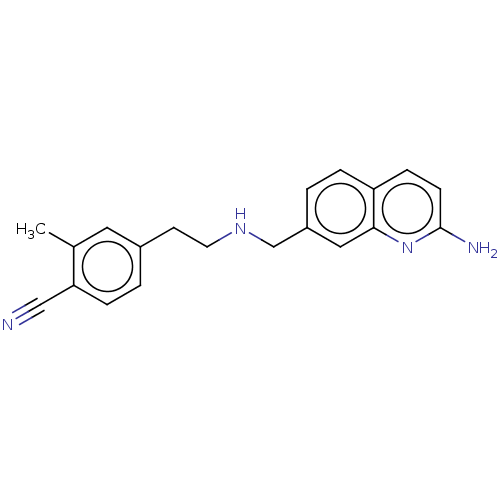 (CHEMBL4068062) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant human nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50258601 (CHEMBL4089246) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant human nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50258602 (CHEMBL4068062) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant rat nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50258687 (CHEMBL4092027) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant rat nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50258687 (CHEMBL4092027) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant human nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50258601 (CHEMBL4089246) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant rat nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50258688 (CHEMBL4062164) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant human nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50258650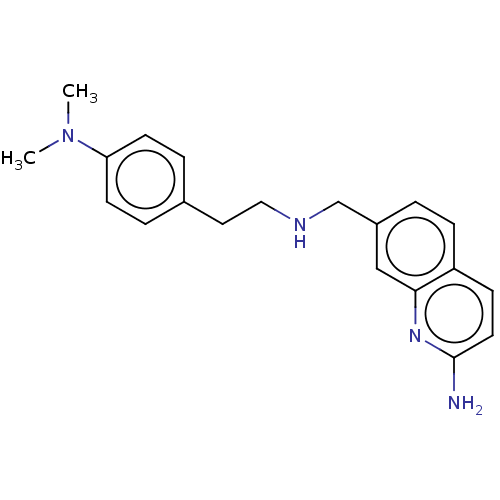 (CHEMBL4100356) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant rat nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50258688 (CHEMBL4062164) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant rat nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50258623 (CHEMBL4071836) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant rat nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50258603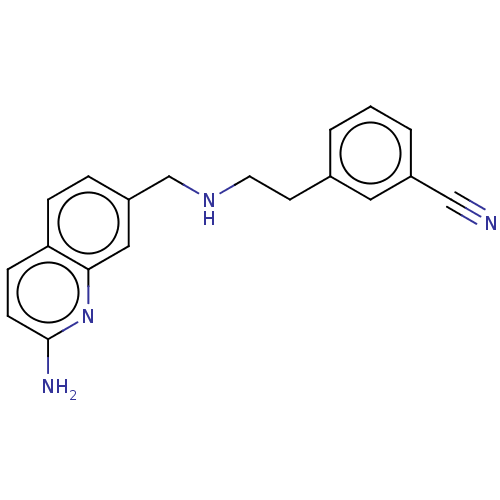 (CHEMBL4090184) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant rat nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50449046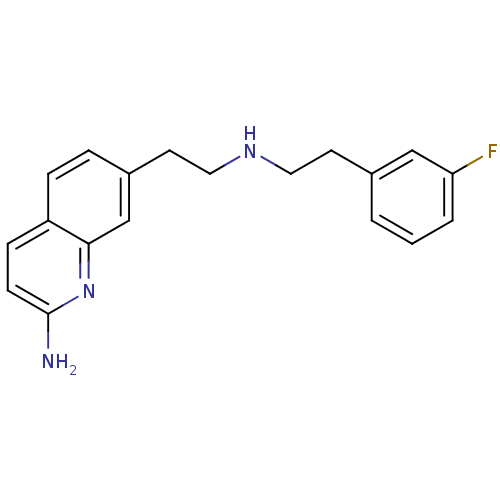 (CHEMBL3126215 | US9212144, 7 (Ex. 13)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant rat nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50258622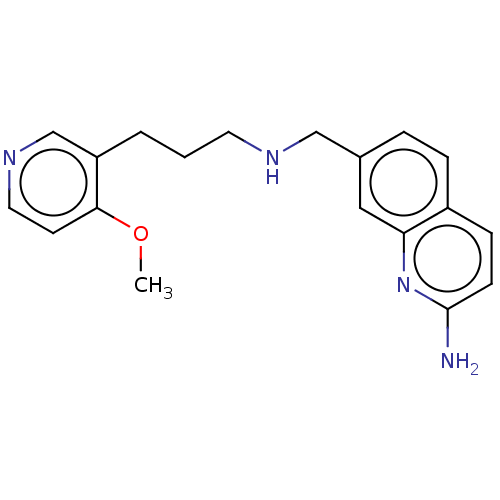 (CHEMBL4097898) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant rat nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50258603 (CHEMBL4090184) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant human nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50258600 (CHEMBL4064297) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant human nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM344422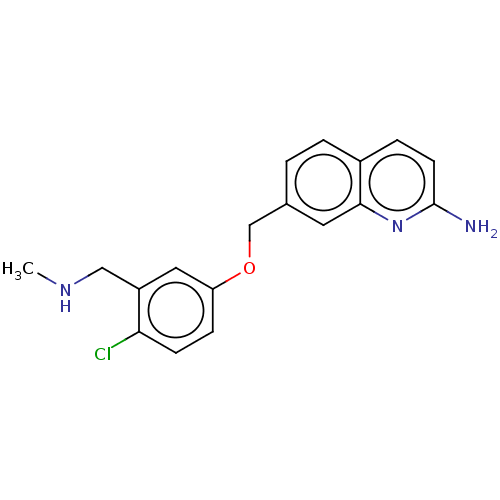 (US9783500, Compound 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant rat nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50449038 (CHEMBL3126204 | US9212144, 15 (Ex. 17)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant rat nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50258622 (CHEMBL4097898) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant human nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50258690 (CHEMBL4082338) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant human nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50258690 (CHEMBL4082338) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant rat nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50258722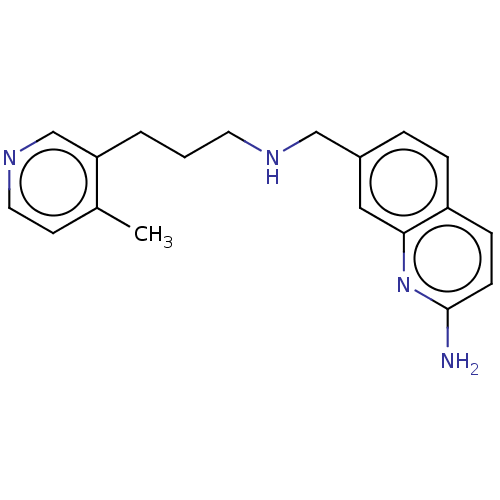 (CHEMBL4092616) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant rat nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50258602 (CHEMBL4068062) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant bovine eNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50449038 (CHEMBL3126204 | US9212144, 15 (Ex. 17)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5-HT4 receptor after 90 mins by scintillation counting method | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50449038 (CHEMBL3126204 | US9212144, 15 (Ex. 17)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Displacement of [3H]GR65630 from human 5-HT3 receptor expressed in HEKT cells after 90 mins by scintillation counting method | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50449038 (CHEMBL3126204 | US9212144, 15 (Ex. 17)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2B receptor expressed in HEK cells after 90 mins by scintillation counting method | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50449038 (CHEMBL3126204 | US9212144, 15 (Ex. 17)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Displacement of [3H]Ketanserin from human 5-HT2A receptor expressed in HEKT cells after 90 mins by scintillation counting method | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50449038 (CHEMBL3126204 | US9212144, 15 (Ex. 17)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Binding affinity to human 5-HT1F receptor | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50449038 (CHEMBL3126204 | US9212144, 15 (Ex. 17)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Displacement of [3H]5-CT from human 5-HT1D receptor expressed in HEKT cells after 90 mins by scintillation counting method | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50449038 (CHEMBL3126204 | US9212144, 15 (Ex. 17)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Displacement of [3H]5-CT from human 5-HT1B receptor expressed in HEK cells after 90 mins by scintillation counting method | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50449038 (CHEMBL3126204 | US9212144, 15 (Ex. 17)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Displacement of [3H]Histamine from human H4 receptor after 90 mins by scintillation counting method | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50449038 (CHEMBL3126204 | US9212144, 15 (Ex. 17)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Displacement of [3H]alpha-methylhistamine from human H3 receptor expressed in HEK Flp-In cells after 90 mins by scintillation counting method | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50449038 (CHEMBL3126204 | US9212144, 15 (Ex. 17)) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human nociceptin opioid receptor expressed in HEK cells after 90 mins by scintillation counting method | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50449038 (CHEMBL3126204 | US9212144, 15 (Ex. 17)) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK cells after 90 mins by scintillation counting method | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50449038 (CHEMBL3126204 | US9212144, 15 (Ex. 17)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Displacement of [3H]Pyrilamine from human H1 receptor expressed in HEK cells after 90 mins by scintillation counting method | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50449038 (CHEMBL3126204 | US9212144, 15 (Ex. 17)) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in HEK cells after 90 mins by scintillation counting method | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50449038 (CHEMBL3126204 | US9212144, 15 (Ex. 17)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT7A receptor expressed in HEK cells after 90 mins by scintillation counting method | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50449038 (CHEMBL3126204 | US9212144, 15 (Ex. 17)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT6 receptor expressed in HEK cells after 90 mins by scintillation counting method | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50449038 (CHEMBL3126204 | US9212144, 15 (Ex. 17)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Displacement of [3H]Way100635 from human 5-HT1a receptor expressed in CHO cells after 90 mins by scintillation counting method | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50449038 (CHEMBL3126204 | US9212144, 15 (Ex. 17)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Displacement of [3H]Tiotidine from human H2 receptor expressed in HEK cells after 90 mins by scintillation counting method | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50449038 (CHEMBL3126204 | US9212144, 15 (Ex. 17)) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in HEK cells after 90 mins by scintillation counting method | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50449038 (CHEMBL3126204 | US9212144, 15 (Ex. 17)) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT5A receptor expressed in Flp-In CHO cells after 90 mins by scintillation counting method | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50449038 (CHEMBL3126204 | US9212144, 15 (Ex. 17)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Displacement of [3H]Mesulergine from human 5-HT2C receptor expressed in Flp-IN HEK cells after 90 mins by scintillation counting method | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM50449038 (CHEMBL3126204 | US9212144, 15 (Ex. 17)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5-HT1E receptor expressed in HEK cells after 90 mins by scintillation counting method | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50449038 (CHEMBL3126204 | US9212144, 15 (Ex. 17)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Displacement of [3H]Way100635 from human 5-HT1A receptor expressed in CHO cells after 90 mins by scintillation counting method | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50449038 (CHEMBL3126204 | US9212144, 15 (Ex. 17)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Displacement of [3H]Tiotidine from human H2 receptor expressed in HEK cells after 90 mins by scintillation counting method | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50258623 (CHEMBL4071836) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 108 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant human nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50258601 (CHEMBL4089246) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant bovine eNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50258722 (CHEMBL4092616) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant human nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50258689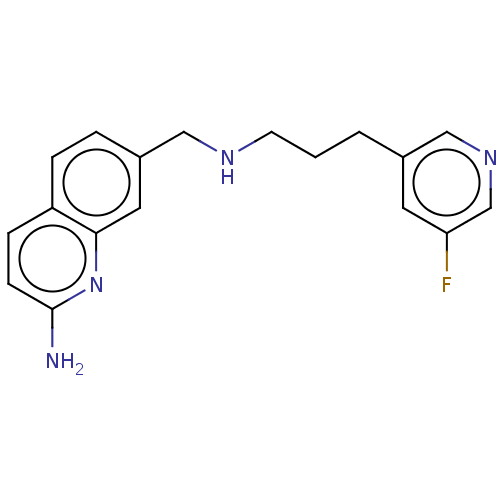 (CHEMBL4070784) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Department of Molecular Biosciences, Chemistry of Life Processes Institute, Center for Molecular Innovation and Drug Discovery, Northwestern University , Evanston, Illinois 6 Curated by ChEMBL | Assay Description Inhibition of recombinant human nNOS expressed in Escherichia coli using L-arginine as substrate after 30 secs by hemoglobin capture assay | J Med Chem 60: 7146-7165 (2017) Article DOI: 10.1021/acs.jmedchem.7b00835 BindingDB Entry DOI: 10.7270/Q2639S64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 110 total ) | Next | Last >> |