 Found 25 hits Enz. Inhib. hit(s) with all data for entry = 50037722
Found 25 hits Enz. Inhib. hit(s) with all data for entry = 50037722 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50186356

(2-(5-(benzyloxy)-6-methoxy-1H-indazol-3-yl)-5-(4-(...)Show SMILES COc1cc2[nH]nc(-c3nc4ccc(cc4[nH]3)N3CCC(CC3)N3CCCCC3)c2cc1OCc1ccccc1 Show InChI InChI=1S/C32H36N6O2/c1-39-29-20-27-25(19-30(29)40-21-22-8-4-2-5-9-22)31(36-35-27)32-33-26-11-10-24(18-28(26)34-32)38-16-12-23(13-17-38)37-14-6-3-7-15-37/h2,4-5,8-11,18-20,23H,3,6-7,12-17,21H2,1H3,(H,33,34)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of c-ABL |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50186360
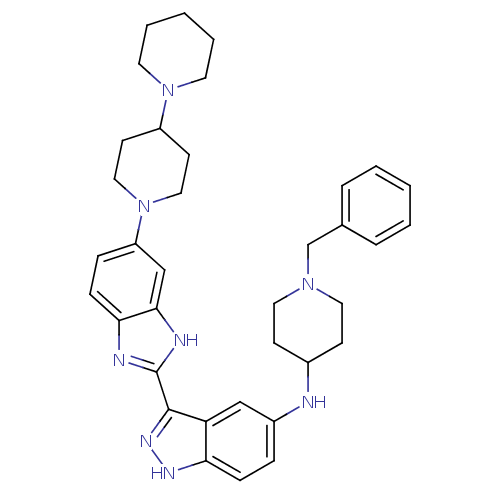
(CHEMBL209753 | N-(1-benzylpiperidin-4-yl)-3-(5-(4-...)Show SMILES C(N1CCC(CC1)Nc1ccc2[nH]nc(-c3nc4ccc(cc4[nH]3)N3CCC(CC3)N3CCCCC3)c2c1)c1ccccc1 Show InChI InChI=1S/C36H44N8/c1-3-7-26(8-4-1)25-42-19-13-27(14-20-42)37-28-9-11-32-31(23-28)35(41-40-32)36-38-33-12-10-30(24-34(33)39-36)44-21-15-29(16-22-44)43-17-5-2-6-18-43/h1,3-4,7-12,23-24,27,29,37H,2,5-6,13-22,25H2,(H,38,39)(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of c-ABL |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50186362
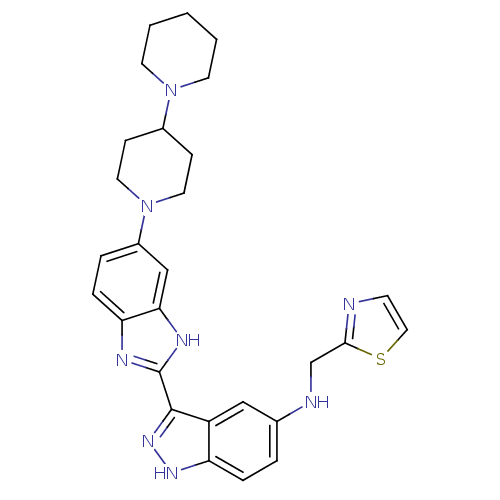
(3-(5-(4-(piperidin-1-yl)piperidin-1-yl)-1H-benzo[d...)Show SMILES C(Nc1ccc2[nH]nc(-c3nc4ccc(cc4[nH]3)N3CCC(CC3)N3CCCCC3)c2c1)c1nccs1 Show InChI InChI=1S/C28H32N8S/c1-2-11-35(12-3-1)20-8-13-36(14-9-20)21-5-7-24-25(17-21)32-28(31-24)27-22-16-19(4-6-23(22)33-34-27)30-18-26-29-10-15-37-26/h4-7,10,15-17,20,30H,1-3,8-9,11-14,18H2,(H,31,32)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of c-ABL |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50186357
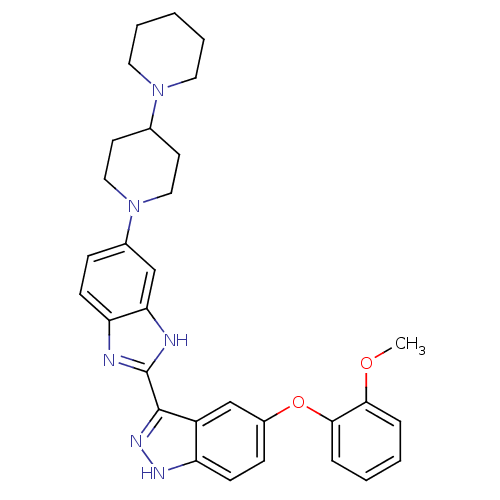
(2-(5-(2-methoxyphenoxy)-1H-indazol-3-yl)-5-(4-(pip...)Show SMILES COc1ccccc1Oc1ccc2[nH]nc(-c3nc4ccc(cc4[nH]3)N3CCC(CC3)N3CCCCC3)c2c1 Show InChI InChI=1S/C31H34N6O2/c1-38-28-7-3-4-8-29(28)39-23-10-12-25-24(20-23)30(35-34-25)31-32-26-11-9-22(19-27(26)33-31)37-17-13-21(14-18-37)36-15-5-2-6-16-36/h3-4,7-12,19-21H,2,5-6,13-18H2,1H3,(H,32,33)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of c-ABL |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50185178

(1-tert-butyl-3-(3-(5-(4-(piperidin-1-yl)piperidin-...)Show SMILES CC(C)(C)NC(=O)Nc1ccc2[nH]nc(-c3nc4ccc(cc4[nH]3)N3CCC(CC3)N3CCCCC3)c2c1 Show InChI InChI=1S/C29H38N8O/c1-29(2,3)33-28(38)30-19-7-9-23-22(17-19)26(35-34-23)27-31-24-10-8-21(18-25(24)32-27)37-15-11-20(12-16-37)36-13-5-4-6-14-36/h7-10,17-18,20H,4-6,11-16H2,1-3H3,(H,31,32)(H,34,35)(H2,30,33,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of c-ABL |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50185169
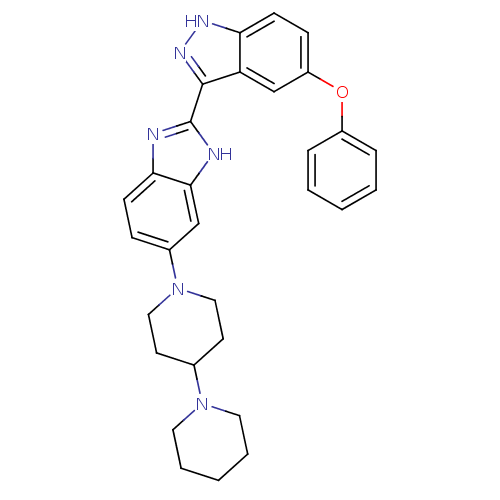
(2-(5-phenoxy-1H-indazol-3-yl)-5-(4-(piperidin-1-yl...)Show SMILES C1CCN(CC1)C1CCN(CC1)c1ccc2nc([nH]c2c1)-c1n[nH]c2ccc(Oc3ccccc3)cc12 Show InChI InChI=1S/C30H32N6O/c1-3-7-23(8-4-1)37-24-10-12-26-25(20-24)29(34-33-26)30-31-27-11-9-22(19-28(27)32-30)36-17-13-21(14-18-36)35-15-5-2-6-16-35/h1,3-4,7-12,19-21H,2,5-6,13-18H2,(H,31,32)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of c-ABL |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50185182
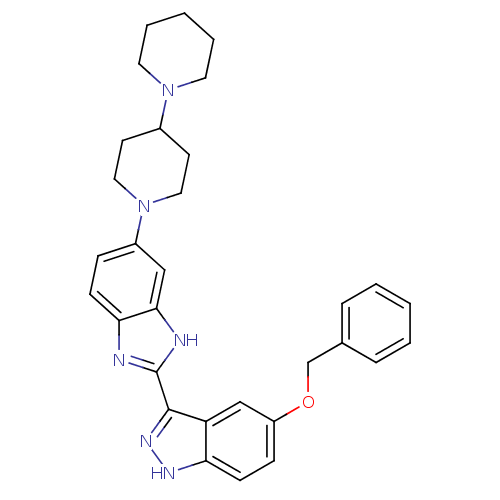
(2-(5-(benzyloxy)-1H-indazol-3-yl)-5-(4-(piperidin-...)Show SMILES C(Oc1ccc2[nH]nc(-c3nc4ccc(cc4[nH]3)N3CCC(CC3)N3CCCCC3)c2c1)c1ccccc1 Show InChI InChI=1S/C31H34N6O/c1-3-7-22(8-4-1)21-38-25-10-12-27-26(20-25)30(35-34-27)31-32-28-11-9-24(19-29(28)33-31)37-17-13-23(14-18-37)36-15-5-2-6-16-36/h1,3-4,7-12,19-20,23H,2,5-6,13-18,21H2,(H,32,33)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of c-ABL |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50186355
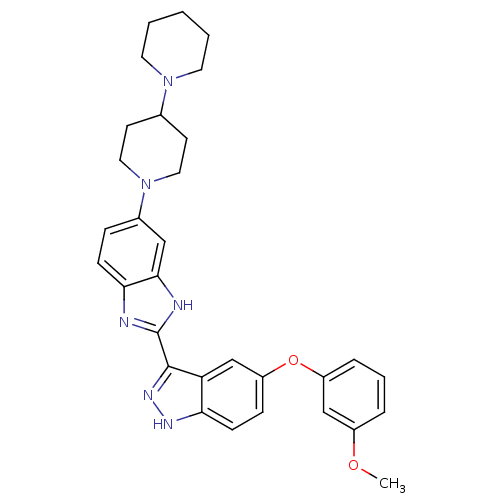
(2-(5-(3-methoxyphenoxy)-1H-indazol-3-yl)-5-(4-(pip...)Show SMILES COc1cccc(Oc2ccc3[nH]nc(-c4nc5ccc(cc5[nH]4)N4CCC(CC4)N4CCCCC4)c3c2)c1 Show InChI InChI=1S/C31H34N6O2/c1-38-23-6-5-7-24(19-23)39-25-9-11-27-26(20-25)30(35-34-27)31-32-28-10-8-22(18-29(28)33-31)37-16-12-21(13-17-37)36-14-3-2-4-15-36/h5-11,18-21H,2-4,12-17H2,1H3,(H,32,33)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of c-ABL |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50186361

(CHEMBL211154 | N-(furan-2-ylmethyl)-3-(5-(4-(piper...)Show SMILES C(Nc1ccc2[nH]nc(-c3nc4ccc(cc4[nH]3)N3CCC(CC3)N3CCCCC3)c2c1)c1ccco1 Show InChI InChI=1S/C29H33N7O/c1-2-12-35(13-3-1)21-10-14-36(15-11-21)22-7-9-26-27(18-22)32-29(31-26)28-24-17-20(6-8-25(24)33-34-28)30-19-23-5-4-16-37-23/h4-9,16-18,21,30H,1-3,10-15,19H2,(H,31,32)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of c-ABL |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50186364

(CHEMBL384213 | N-benzyl-3-(5-(4-(piperidin-1-yl)pi...)Show SMILES C(Nc1ccc2[nH]nc(-c3nc4ccc(cc4[nH]3)N3CCC(CC3)N3CCCCC3)c2c1)c1ccccc1 Show InChI InChI=1S/C31H35N7/c1-3-7-22(8-4-1)21-32-23-9-11-27-26(19-23)30(36-35-27)31-33-28-12-10-25(20-29(28)34-31)38-17-13-24(14-18-38)37-15-5-2-6-16-37/h1,3-4,7-12,19-20,24,32H,2,5-6,13-18,21H2,(H,33,34)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of c-ABL |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50186358

(CHEMBL377463 | N-(3-(5-(4-(piperidin-1-yl)piperidi...)Show SMILES O=C(Nc1ccc2[nH]nc(-c3nc4ccc(cc4[nH]3)N3CCC(CC3)N3CCCCC3)c2c1)c1ccco1 Show InChI InChI=1S/C29H31N7O2/c37-29(26-5-4-16-38-26)30-19-6-8-23-22(17-19)27(34-33-23)28-31-24-9-7-21(18-25(24)32-28)36-14-10-20(11-15-36)35-12-2-1-3-13-35/h4-9,16-18,20H,1-3,10-15H2,(H,30,37)(H,31,32)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of c-ABL |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50185184
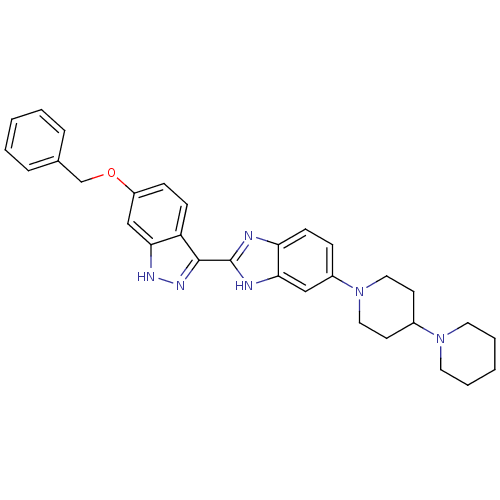
(2-(6-(benzyloxy)-1H-indazol-3-yl)-5-(4-(piperidin-...)Show SMILES C(Oc1ccc2c(n[nH]c2c1)-c1nc2ccc(cc2[nH]1)N1CCC(CC1)N1CCCCC1)c1ccccc1 Show InChI InChI=1S/C31H34N6O/c1-3-7-22(8-4-1)21-38-25-10-11-26-28(20-25)34-35-30(26)31-32-27-12-9-24(19-29(27)33-31)37-17-13-23(14-18-37)36-15-5-2-6-16-36/h1,3-4,7-12,19-20,23H,2,5-6,13-18,21H2,(H,32,33)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of c-ABL |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50186363

(2-(5-(4-methoxyphenoxy)-1H-indazol-3-yl)-5-(4-(pip...)Show SMILES COc1ccc(Oc2ccc3[nH]nc(-c4nc5ccc(cc5[nH]4)N4CCC(CC4)N4CCCCC4)c3c2)cc1 Show InChI InChI=1S/C31H34N6O2/c1-38-23-6-8-24(9-7-23)39-25-10-12-27-26(20-25)30(35-34-27)31-32-28-11-5-22(19-29(28)33-31)37-17-13-21(14-18-37)36-15-3-2-4-16-36/h5-12,19-21H,2-4,13-18H2,1H3,(H,32,33)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of c-ABL |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50186359
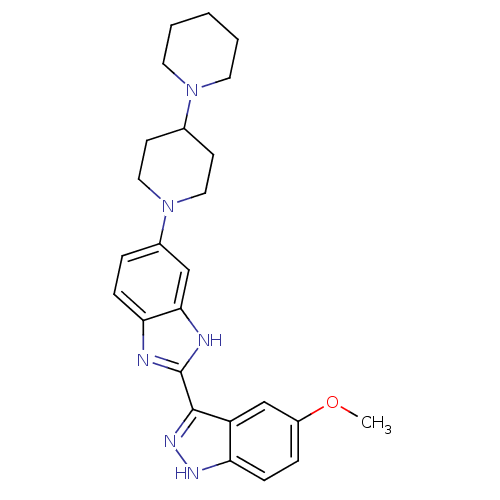
(2-(5-methoxy-1H-indazol-3-yl)-5-(4-(piperidin-1-yl...)Show SMILES COc1ccc2[nH]nc(-c3nc4ccc(cc4[nH]3)N3CCC(CC3)N3CCCCC3)c2c1 Show InChI InChI=1S/C25H30N6O/c1-32-19-6-8-21-20(16-19)24(29-28-21)25-26-22-7-5-18(15-23(22)27-25)31-13-9-17(10-14-31)30-11-3-2-4-12-30/h5-8,15-17H,2-4,9-14H2,1H3,(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of c-ABL |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of c-ABL |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50185170
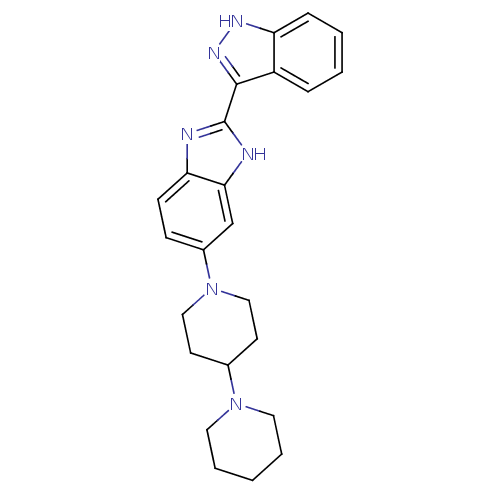
(2-(1H-indazol-3-yl)-5-(4-(piperidin-1-yl)piperidin...)Show SMILES C1CCN(CC1)C1CCN(CC1)c1ccc2nc([nH]c2c1)-c1n[nH]c2ccccc12 Show InChI InChI=1S/C24H28N6/c1-4-12-29(13-5-1)17-10-14-30(15-11-17)18-8-9-21-22(16-18)26-24(25-21)23-19-6-2-3-7-20(19)27-28-23/h2-3,6-9,16-17H,1,4-5,10-15H2,(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of c-ABL |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50186365
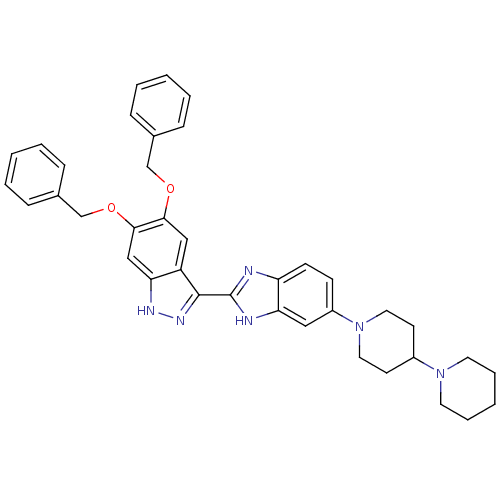
(2-(5,6-bis(benzyloxy)-1H-indazol-3-yl)-5-(4-(piper...)Show SMILES C(Oc1cc2[nH]nc(-c3nc4ccc(cc4[nH]3)N3CCC(CC3)N3CCCCC3)c2cc1OCc1ccccc1)c1ccccc1 Show InChI InChI=1S/C38H40N6O2/c1-4-10-27(11-5-1)25-45-35-23-31-33(24-36(35)46-26-28-12-6-2-7-13-28)41-42-37(31)38-39-32-15-14-30(22-34(32)40-38)44-20-16-29(17-21-44)43-18-8-3-9-19-43/h1-2,4-7,10-15,22-24,29H,3,8-9,16-21,25-26H2,(H,39,40)(H,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of c-ABL |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | |
Breakpoint cluster region protein/Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Antiproliferative activity against K562 cells expressing Bcr-Abl |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Breakpoint cluster region protein/Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50186360

(CHEMBL209753 | N-(1-benzylpiperidin-4-yl)-3-(5-(4-...)Show SMILES C(N1CCC(CC1)Nc1ccc2[nH]nc(-c3nc4ccc(cc4[nH]3)N3CCC(CC3)N3CCCCC3)c2c1)c1ccccc1 Show InChI InChI=1S/C36H44N8/c1-3-7-26(8-4-1)25-42-19-13-27(14-20-42)37-28-9-11-32-31(23-28)35(41-40-32)36-38-33-12-10-30(24-34(33)39-36)44-21-15-29(16-22-44)43-17-5-2-6-18-43/h1,3-4,7-12,23-24,27,29,37H,2,5-6,13-22,25H2,(H,38,39)(H,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Antiproliferative activity against K562 cells expressing Bcr-Abl |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | |
Breakpoint cluster region protein/Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50186362

(3-(5-(4-(piperidin-1-yl)piperidin-1-yl)-1H-benzo[d...)Show SMILES C(Nc1ccc2[nH]nc(-c3nc4ccc(cc4[nH]3)N3CCC(CC3)N3CCCCC3)c2c1)c1nccs1 Show InChI InChI=1S/C28H32N8S/c1-2-11-35(12-3-1)20-8-13-36(14-9-20)21-5-7-24-25(17-21)32-28(31-24)27-22-16-19(4-6-23(22)33-34-27)30-18-26-29-10-15-37-26/h4-7,10,15-17,20,30H,1-3,8-9,11-14,18H2,(H,31,32)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Antiproliferative activity against K562 cells expressing Bcr-Abl |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | |
Breakpoint cluster region protein/Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50186358

(CHEMBL377463 | N-(3-(5-(4-(piperidin-1-yl)piperidi...)Show SMILES O=C(Nc1ccc2[nH]nc(-c3nc4ccc(cc4[nH]3)N3CCC(CC3)N3CCCCC3)c2c1)c1ccco1 Show InChI InChI=1S/C29H31N7O2/c37-29(26-5-4-16-38-26)30-19-6-8-23-22(17-19)27(34-33-23)28-31-24-9-7-21(18-25(24)32-28)36-14-10-20(11-15-36)35-12-2-1-3-13-35/h4-9,16-18,20H,1-3,10-15H2,(H,30,37)(H,31,32)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 560 | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Antiproliferative activity against K562 cells expressing Bcr-Abl |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | |
Breakpoint cluster region protein/Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50185178

(1-tert-butyl-3-(3-(5-(4-(piperidin-1-yl)piperidin-...)Show SMILES CC(C)(C)NC(=O)Nc1ccc2[nH]nc(-c3nc4ccc(cc4[nH]3)N3CCC(CC3)N3CCCCC3)c2c1 Show InChI InChI=1S/C29H38N8O/c1-29(2,3)33-28(38)30-19-7-9-23-22(17-19)26(35-34-23)27-31-24-10-8-21(18-25(24)32-27)37-15-11-20(12-16-37)36-13-5-4-6-14-36/h7-10,17-18,20H,4-6,11-16H2,1-3H3,(H,31,32)(H,34,35)(H2,30,33,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 360 | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Antiproliferative activity against K562 cells expressing Bcr-Abl |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | |
Breakpoint cluster region protein/Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50185182

(2-(5-(benzyloxy)-1H-indazol-3-yl)-5-(4-(piperidin-...)Show SMILES C(Oc1ccc2[nH]nc(-c3nc4ccc(cc4[nH]3)N3CCC(CC3)N3CCCCC3)c2c1)c1ccccc1 Show InChI InChI=1S/C31H34N6O/c1-3-7-22(8-4-1)21-38-25-10-12-27-26(20-25)30(35-34-27)31-32-28-11-9-24(19-29(28)33-31)37-17-13-23(14-18-37)36-15-5-2-6-16-36/h1,3-4,7-12,19-20,23H,2,5-6,13-18,21H2,(H,32,33)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 970 | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Antiproliferative activity against K562 cells expressing Bcr-Abl |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | |
Breakpoint cluster region protein/Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50186361

(CHEMBL211154 | N-(furan-2-ylmethyl)-3-(5-(4-(piper...)Show SMILES C(Nc1ccc2[nH]nc(-c3nc4ccc(cc4[nH]3)N3CCC(CC3)N3CCCCC3)c2c1)c1ccco1 Show InChI InChI=1S/C29H33N7O/c1-2-12-35(13-3-1)21-10-14-36(15-11-21)22-7-9-26-27(18-22)32-29(31-26)28-24-17-20(6-8-25(24)33-34-28)30-19-23-5-4-16-37-23/h4-9,16-18,21,30H,1-3,10-15,19H2,(H,31,32)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Antiproliferative activity against K562 cells expressing Bcr-Abl |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | |
Breakpoint cluster region protein/Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50186364

(CHEMBL384213 | N-benzyl-3-(5-(4-(piperidin-1-yl)pi...)Show SMILES C(Nc1ccc2[nH]nc(-c3nc4ccc(cc4[nH]3)N3CCC(CC3)N3CCCCC3)c2c1)c1ccccc1 Show InChI InChI=1S/C31H35N7/c1-3-7-22(8-4-1)21-32-23-9-11-27-26(19-23)30(36-35-27)31-33-28-12-10-25(20-29(28)34-31)38-17-13-24(14-18-38)37-15-5-2-6-16-37/h1,3-4,7-12,19-20,24,32H,2,5-6,13-18,21H2,(H,33,34)(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Antiproliferative activity against K562 cells expressing Bcr-Abl |
Bioorg Med Chem Lett 16: 3789-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.043
BindingDB Entry DOI: 10.7270/Q2HD7WG3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data