

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50266734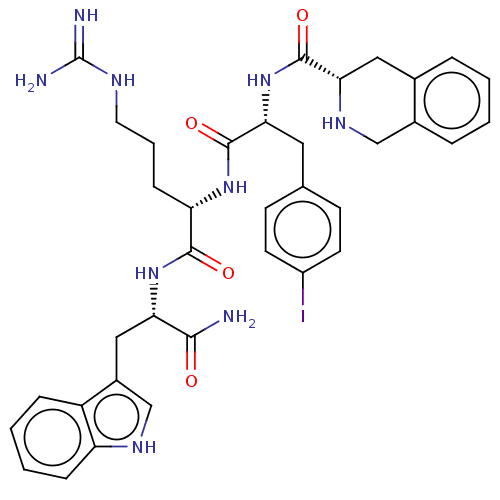 (CHEMBL4096081) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Antagonist activity at human MC4R expressed in CHO cells assessed as inhibition of aplha MSH-induced cAMP activation after 45 mins | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266704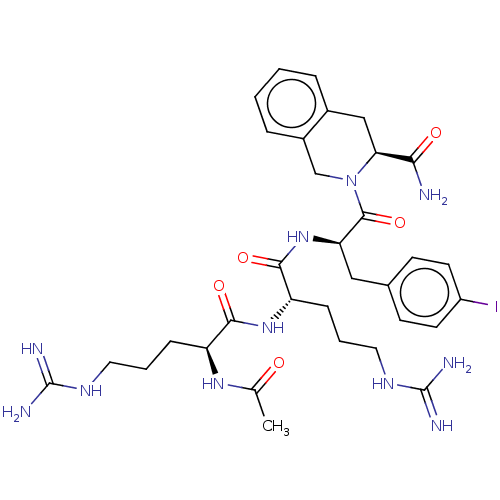 (CHEMBL4083717) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Antagonist activity at mouse MC4R expressed in HEK293 cells assessed as inhibition of NDP-MSH induced-cAMP accumulation after 2 hrs by alpha screen a... | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266713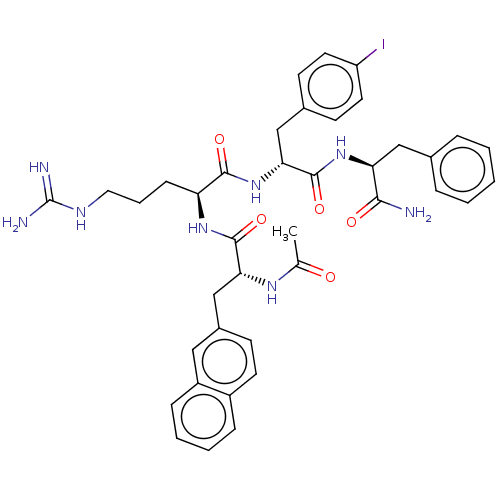 (CHEMBL4089759) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Antagonist activity at mouse MC4R expressed in HEK293 cells assessed as inhibition of NDP-MSH induced-cAMP accumulation after 2 hrs by alpha screen a... | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50017181 (CHEMBL441738) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-MSH from mouse MC4R expressed in HEK293 cells after 1 hr by gamma counting | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50017181 (CHEMBL441738) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-MSH from mouse MC3R expressed in HEK293 cells after 1 hr by gamma counting | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266704 (CHEMBL4083717) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-MSH from mouse MC4R expressed in HEK293 cells after 1 hr by gamma counting | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266726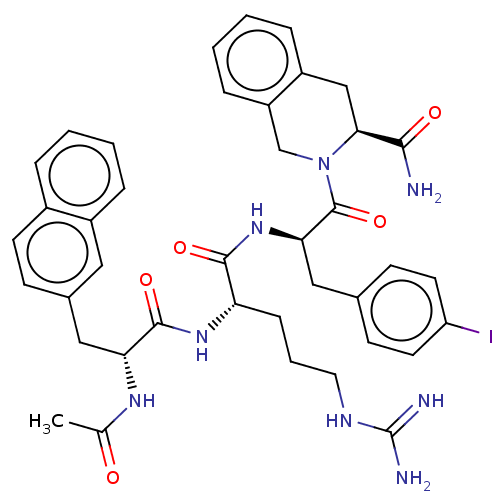 (CHEMBL4078495) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-MSH from mouse MC4R expressed in HEK293 cells after 1 hr by gamma counting | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266696 (CHEMBL4063911) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-MSH from mouse MC4R expressed in HEK293 cells after 1 hr by gamma counting | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266739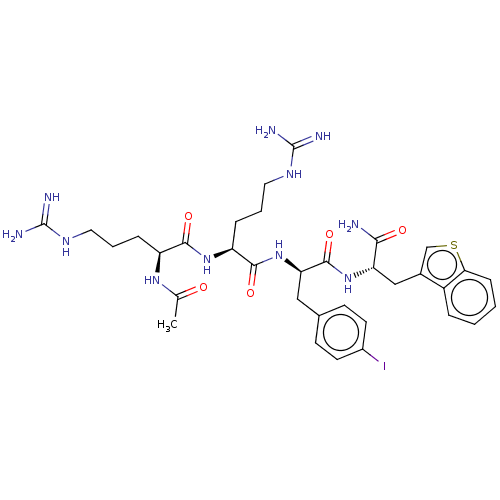 (CHEMBL4071063) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-MSH from mouse MC4R expressed in HEK293 cells after 1 hr by gamma counting | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50034922 ((S)-2-{(R)-2-[(S)-2-Acetylamino-3-(1H-imidazol-4-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-MSH from mouse MC4R expressed in HEK293 cells after 1 hr by gamma counting | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266706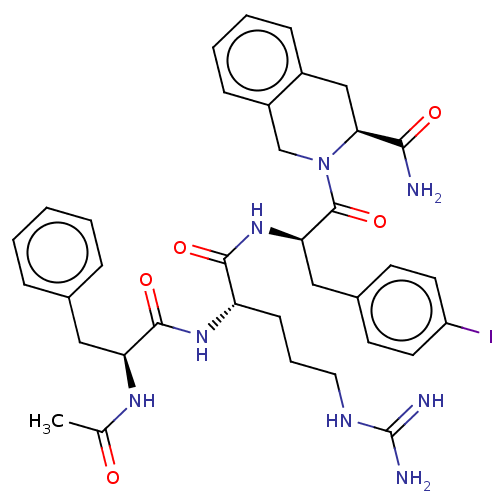 (CHEMBL4079298) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-MSH from mouse MC4R expressed in HEK293 cells after 1 hr by gamma counting | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266722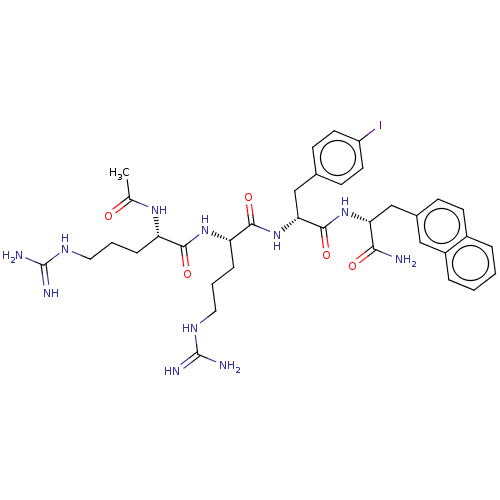 (CHEMBL4072129) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-MSH from mouse MC4R expressed in HEK293 cells after 1 hr by gamma counting | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266740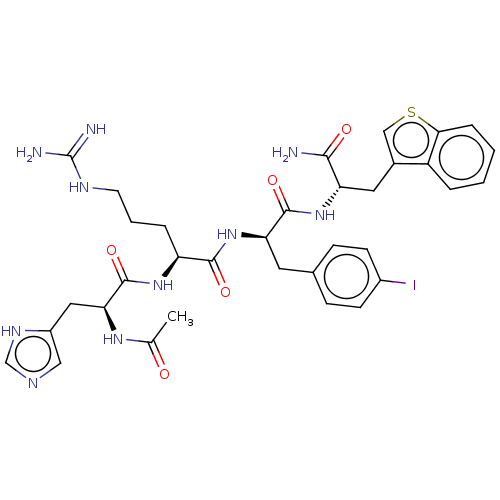 (CHEMBL4091894) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-MSH from mouse MC4R expressed in HEK293 cells after 1 hr by gamma counting | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50266726 (CHEMBL4078495) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-MSH from mouse MC3R expressed in HEK293 cells after 1 hr by gamma counting | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50266722 (CHEMBL4072129) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-MSH from mouse MC3R expressed in HEK293 cells after 1 hr by gamma counting | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266708 (CHEMBL4102774) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 455 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-MSH from mouse MC4R expressed in HEK293 cells after 1 hr by gamma counting | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50266704 (CHEMBL4083717) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-MSH from mouse MC3R expressed in HEK293 cells after 1 hr by gamma counting | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266731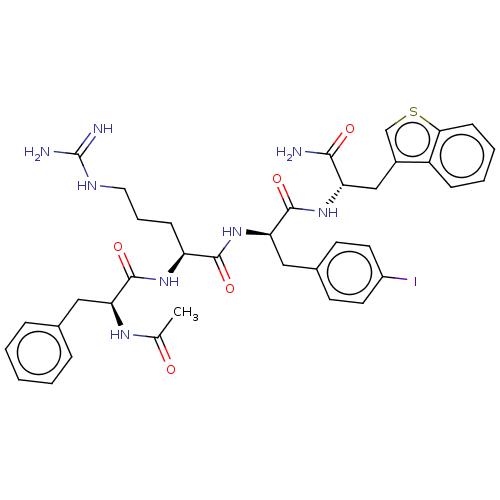 (CHEMBL4092424) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-MSH from mouse MC4R expressed in HEK293 cells after 1 hr by gamma counting | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50266739 (CHEMBL4071063) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 965 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-MSH from mouse MC3R expressed in HEK293 cells after 1 hr by gamma counting | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50266696 (CHEMBL4063911) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 975 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-MSH from mouse MC3R expressed in HEK293 cells after 1 hr by gamma counting | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50266706 (CHEMBL4079298) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-MSH from mouse MC3R expressed in HEK293 cells after 1 hr by gamma counting | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50266740 (CHEMBL4091894) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-MSH from mouse MC3R expressed in HEK293 cells after 1 hr by gamma counting | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50266731 (CHEMBL4092424) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-MSH from mouse MC3R expressed in HEK293 cells after 1 hr by gamma counting | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50266708 (CHEMBL4102774) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-MSH from mouse MC3R expressed in HEK293 cells after 1 hr by gamma counting | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50034922 ((S)-2-{(R)-2-[(S)-2-Acetylamino-3-(1H-imidazol-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-MSH from mouse MC3R expressed in HEK293 cells after 1 hr by gamma counting | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266696 (CHEMBL4063911) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC4R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266697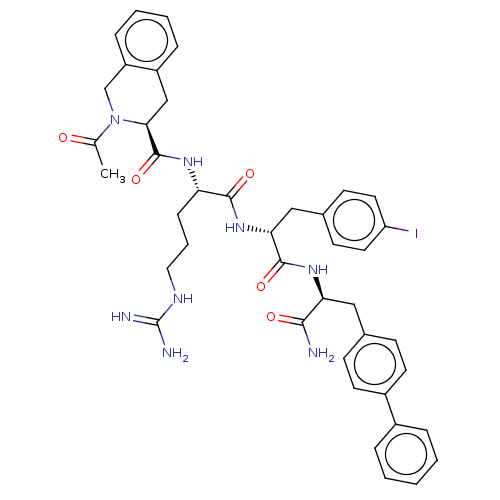 (CHEMBL4100584) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC4R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266698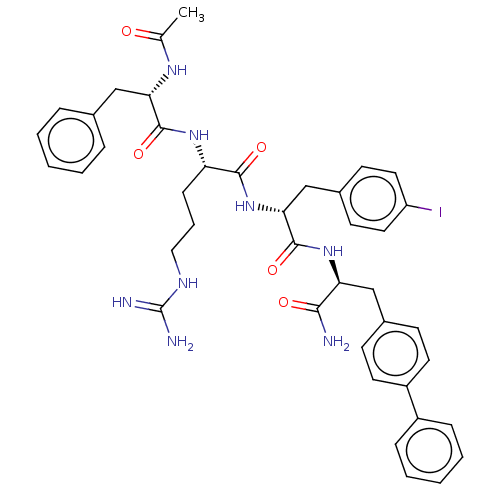 (CHEMBL4073782) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC4R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266699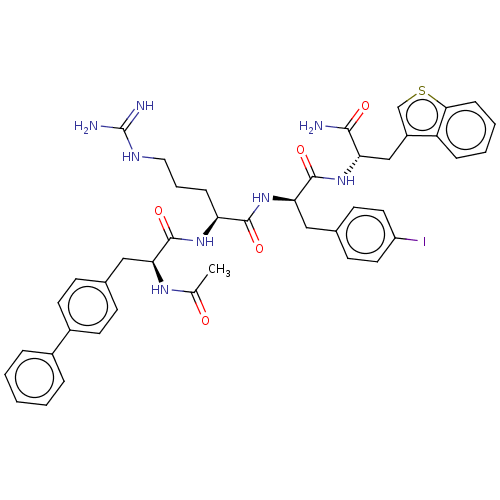 (CHEMBL4103985) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC4R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266700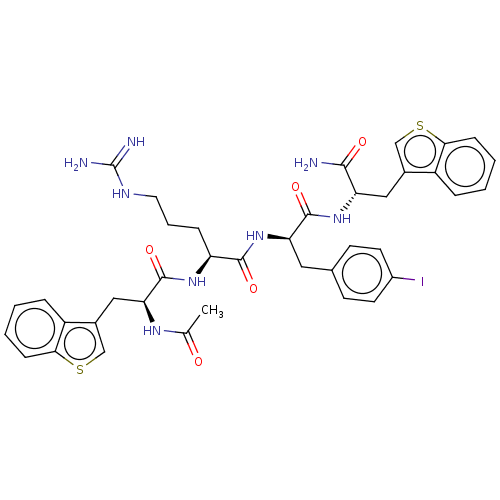 (CHEMBL4079721) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC4R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266701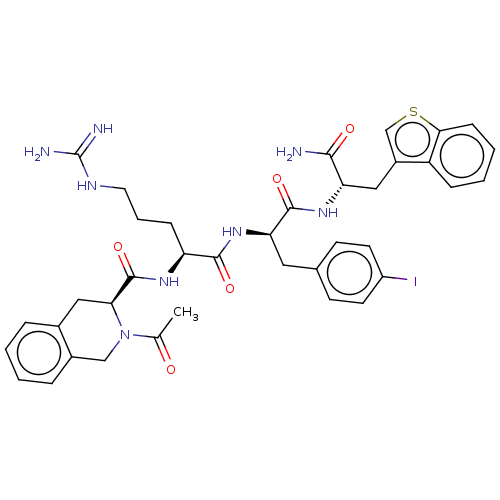 (CHEMBL4064728) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC4R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266702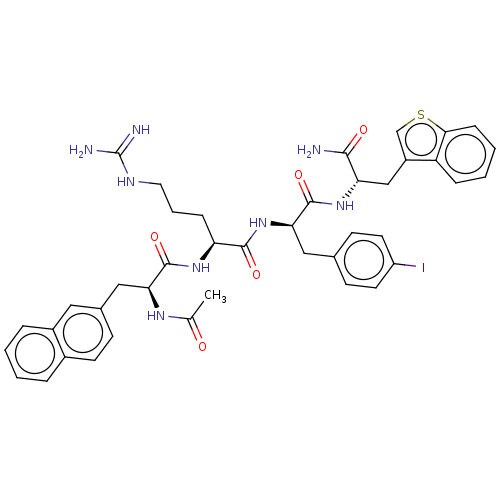 (CHEMBL4102613) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC4R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266704 (CHEMBL4083717) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC4R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266706 (CHEMBL4079298) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC4R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266708 (CHEMBL4102774) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC4R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266709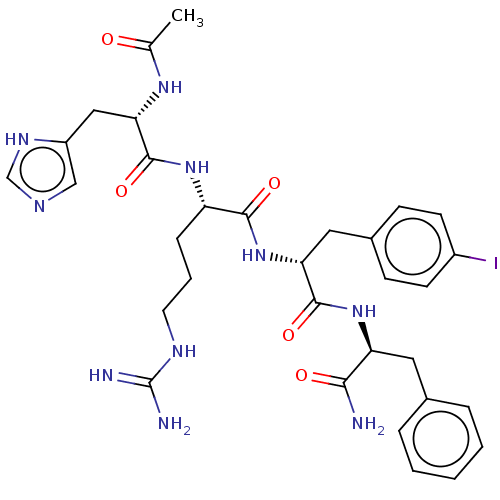 (CHEMBL4086346) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC4R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266710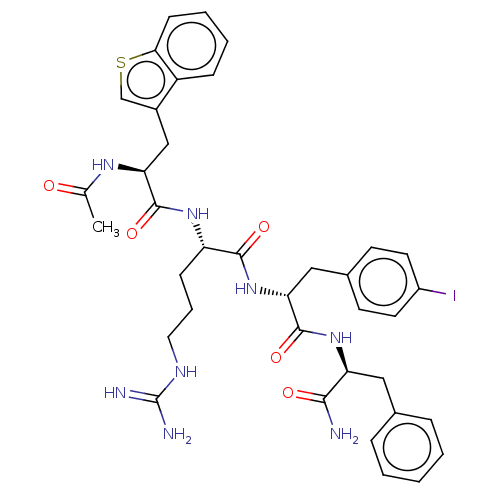 (CHEMBL4094121) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC4R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266711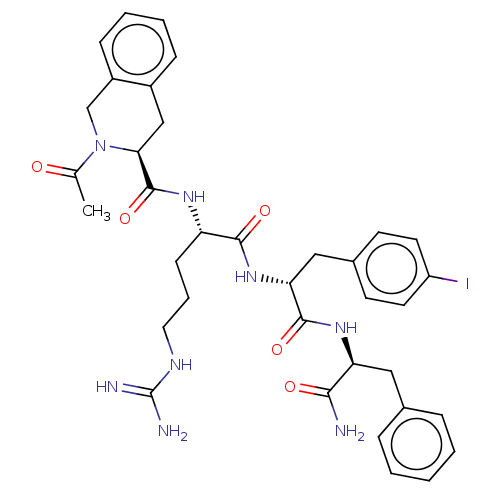 (CHEMBL4073480) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC4R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266712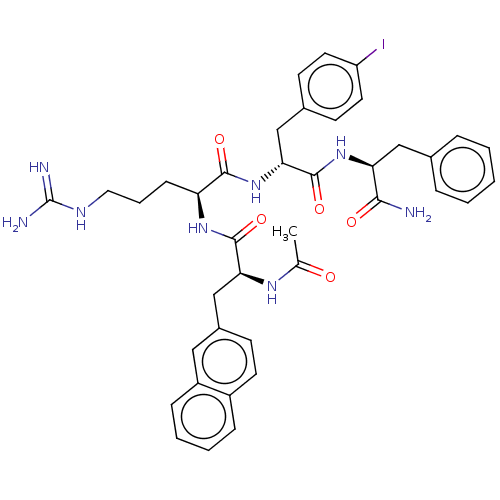 (CHEMBL4083857) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC4R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266713 (CHEMBL4089759) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC4R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266714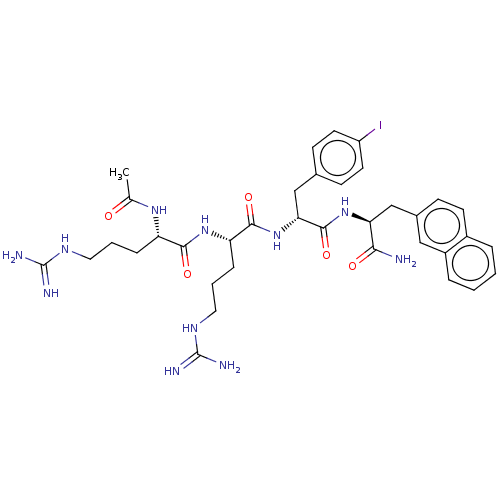 (CHEMBL4068643) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC4R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266715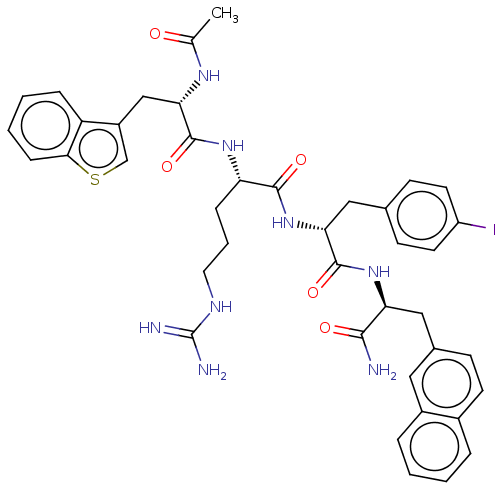 (CHEMBL4069415) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC4R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266716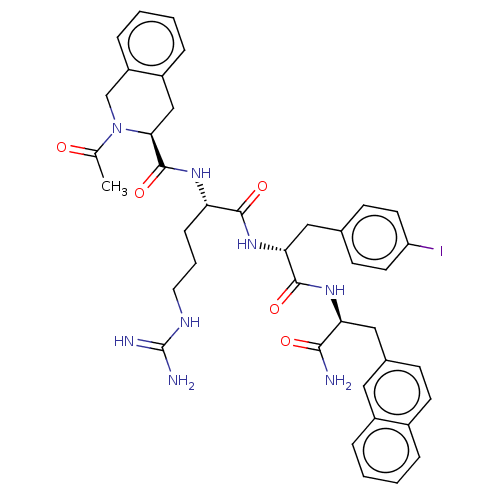 (CHEMBL4090448) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC4R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266717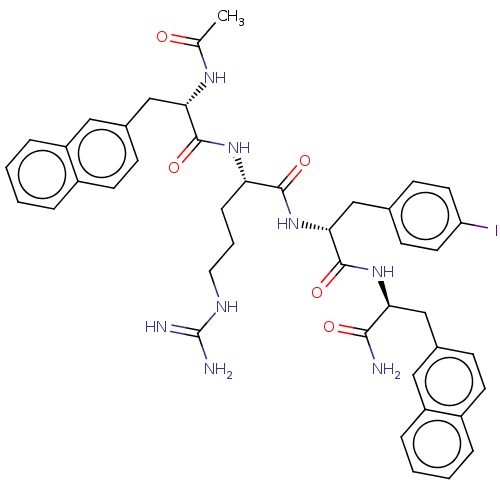 (CHEMBL4082615) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC4R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266722 (CHEMBL4072129) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC4R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266723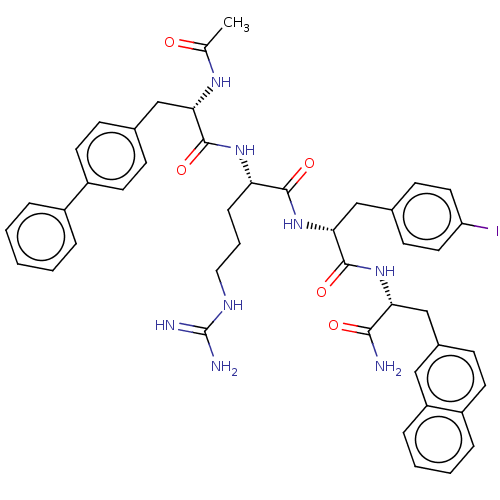 (CHEMBL4087093) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC4R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50266724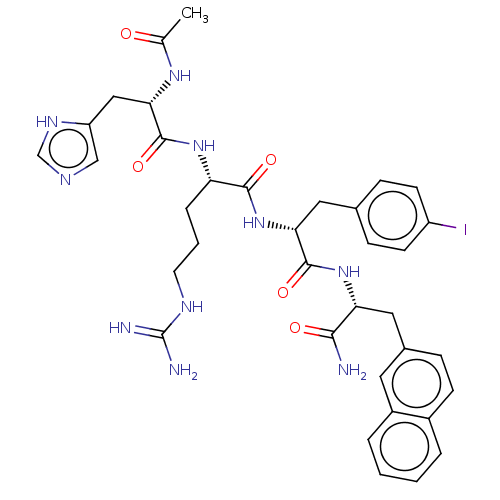 (CHEMBL4065669) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC3R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50266722 (CHEMBL4072129) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 57 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC3R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50266716 (CHEMBL4090448) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC3R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Mus musculus) | BDBM50266725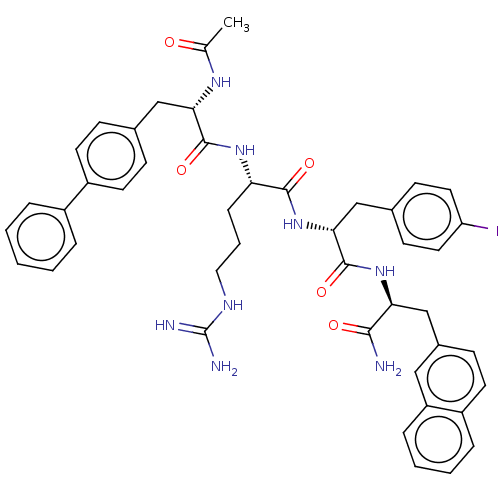 (CHEMBL4098164) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Agonist activity at mouse MC3R expressed in HEK293 cells assessed as induction of cAMP accumulation after 2 hrs by alpha screen assay | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 163 total ) | Next | Last >> |