

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50380808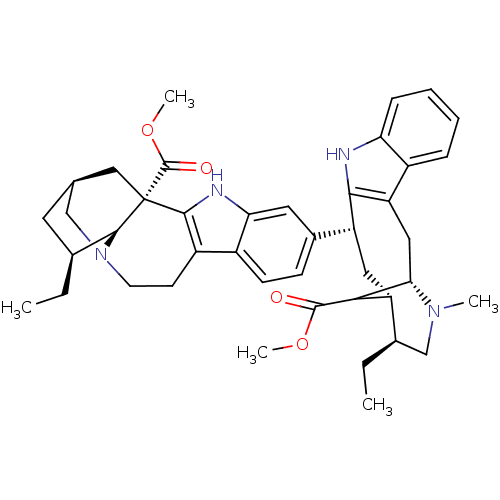 (CHEMBL2018162) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Naresuan University Curated by ChEMBL | Assay Description Inhibition of AChE by Ellman's method | Bioorg Med Chem Lett 22: 2885-8 (2012) Article DOI: 10.1016/j.bmcl.2012.02.057 BindingDB Entry DOI: 10.7270/Q2251K5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50380807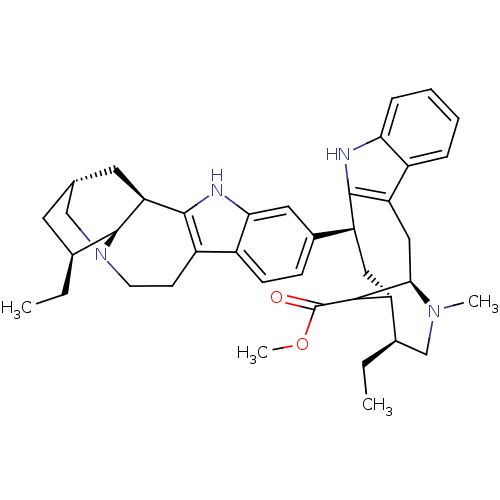 (CHEMBL376478) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 227 | n/a | n/a | n/a | n/a | n/a | n/a |
Naresuan University Curated by ChEMBL | Assay Description Inhibition of AChE by Ellman's method | Bioorg Med Chem Lett 22: 2885-8 (2012) Article DOI: 10.1016/j.bmcl.2012.02.057 BindingDB Entry DOI: 10.7270/Q2251K5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Naresuan University Curated by ChEMBL | Assay Description Inhibition of human AChE by Ellman's method | Bioorg Med Chem Lett 22: 2885-8 (2012) Article DOI: 10.1016/j.bmcl.2012.02.057 BindingDB Entry DOI: 10.7270/Q2251K5M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Naresuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine iodide as substrate measured every 5 sec for 2 mins by Ellman's method | Bioorg Med Chem Lett 22: 2885-8 (2012) Article DOI: 10.1016/j.bmcl.2012.02.057 BindingDB Entry DOI: 10.7270/Q2251K5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50380806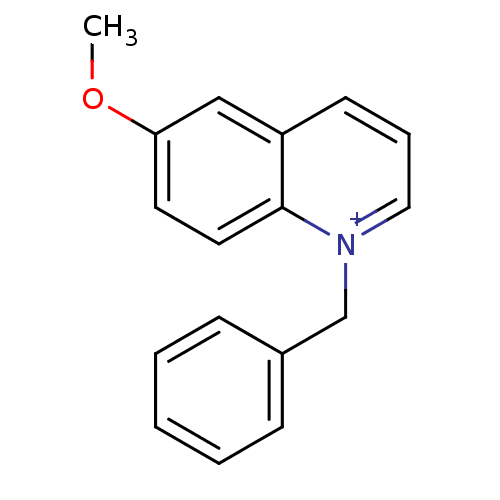 (CHEMBL2018161) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Naresuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine iodide as substrate measured every 5 sec for 2 mins by Ellman's method | Bioorg Med Chem Lett 22: 2885-8 (2012) Article DOI: 10.1016/j.bmcl.2012.02.057 BindingDB Entry DOI: 10.7270/Q2251K5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50029799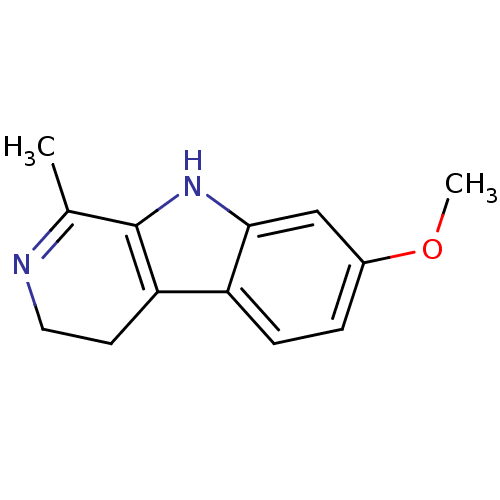 (7-Methoxy-1-methyl-2,9-dihydro-1H-beta-carboline |...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Naresuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine iodide as substrate measured every 5 sec for 2 mins by Ellman's method | Bioorg Med Chem Lett 22: 2885-8 (2012) Article DOI: 10.1016/j.bmcl.2012.02.057 BindingDB Entry DOI: 10.7270/Q2251K5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50380806 (CHEMBL2018161) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Naresuan University Curated by ChEMBL | Assay Description Inhibition of human AChE by Ellman's method | Bioorg Med Chem Lett 22: 2885-8 (2012) Article DOI: 10.1016/j.bmcl.2012.02.057 BindingDB Entry DOI: 10.7270/Q2251K5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50380805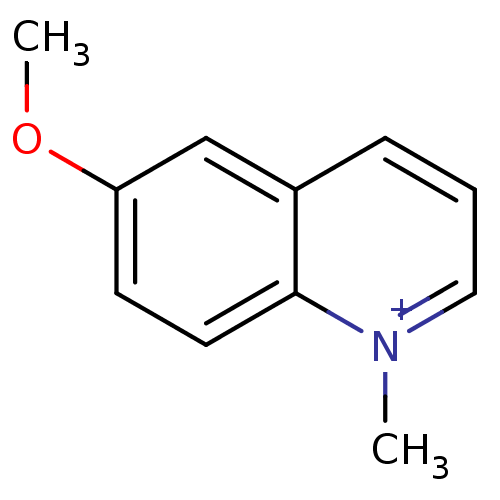 (CHEMBL2018160) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Naresuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine iodide as substrate measured every 5 sec for 2 mins by Ellman's method | Bioorg Med Chem Lett 22: 2885-8 (2012) Article DOI: 10.1016/j.bmcl.2012.02.057 BindingDB Entry DOI: 10.7270/Q2251K5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50047009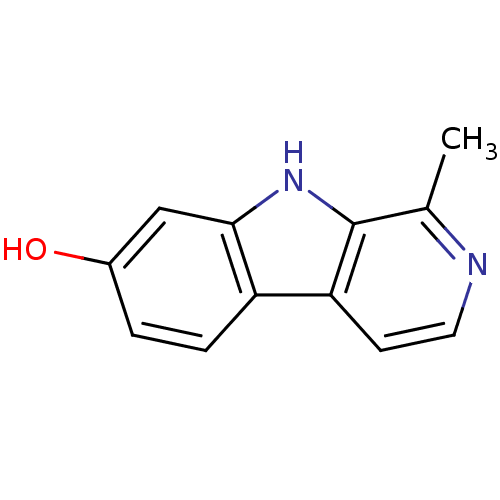 (1-Methyl-9H-beta-carbolin-7-ol | 1-Methyl-9H-beta-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Naresuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine iodide as substrate measured every 5 sec for 2 mins by Ellman's method | Bioorg Med Chem Lett 22: 2885-8 (2012) Article DOI: 10.1016/j.bmcl.2012.02.057 BindingDB Entry DOI: 10.7270/Q2251K5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50132101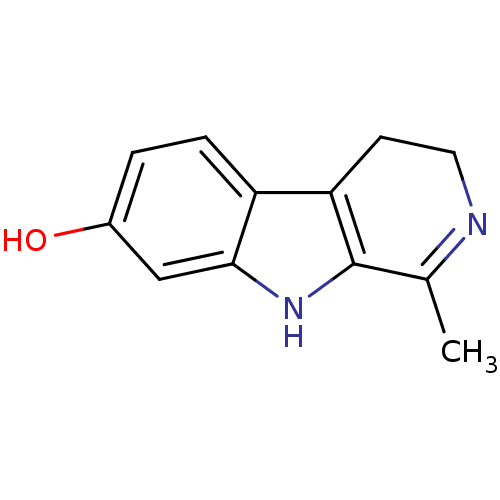 (1-Methyl-4,9-dihydro-3H-beta-carbolin-7-ol | CHEMB...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Naresuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine iodide as substrate measured every 5 sec for 2 mins by Ellman's method | Bioorg Med Chem Lett 22: 2885-8 (2012) Article DOI: 10.1016/j.bmcl.2012.02.057 BindingDB Entry DOI: 10.7270/Q2251K5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50013786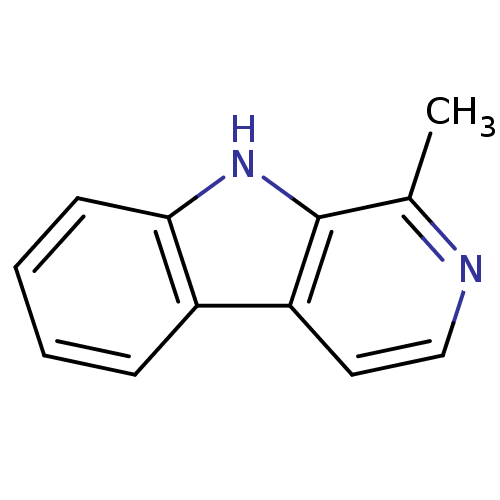 (1-Methyl-9H-beta-carboline | 1-Methyl-9H-beta-carb...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Naresuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine iodide as substrate measured every 5 sec for 2 mins by Ellman's method | Bioorg Med Chem Lett 22: 2885-8 (2012) Article DOI: 10.1016/j.bmcl.2012.02.057 BindingDB Entry DOI: 10.7270/Q2251K5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM100152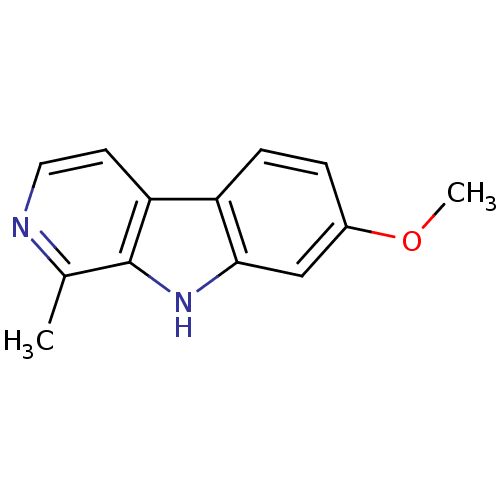 (7-methoxy-1-methyl-9H-beta-carboline;hydrochloride...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Naresuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine iodide as substrate measured every 5 sec for 2 mins by Ellman's method | Bioorg Med Chem Lett 22: 2885-8 (2012) Article DOI: 10.1016/j.bmcl.2012.02.057 BindingDB Entry DOI: 10.7270/Q2251K5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50029799 (7-Methoxy-1-methyl-2,9-dihydro-1H-beta-carboline |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Naresuan University Curated by ChEMBL | Assay Description Inhibition of human AChE by Ellman's method | Bioorg Med Chem Lett 22: 2885-8 (2012) Article DOI: 10.1016/j.bmcl.2012.02.057 BindingDB Entry DOI: 10.7270/Q2251K5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50380805 (CHEMBL2018160) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Naresuan University Curated by ChEMBL | Assay Description Inhibition of human AChE by Ellman's method | Bioorg Med Chem Lett 22: 2885-8 (2012) Article DOI: 10.1016/j.bmcl.2012.02.057 BindingDB Entry DOI: 10.7270/Q2251K5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50130262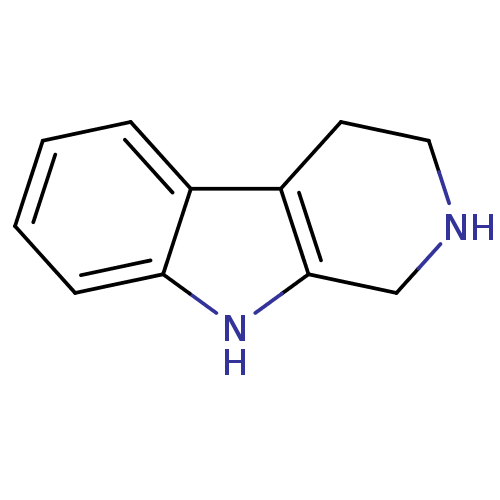 (2,3,4,9-Tetrahydro-1H-beta-carboline | CHEMBL26923...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Naresuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine iodide as substrate measured every 5 sec for 2 mins by Ellman's method | Bioorg Med Chem Lett 22: 2885-8 (2012) Article DOI: 10.1016/j.bmcl.2012.02.057 BindingDB Entry DOI: 10.7270/Q2251K5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50136492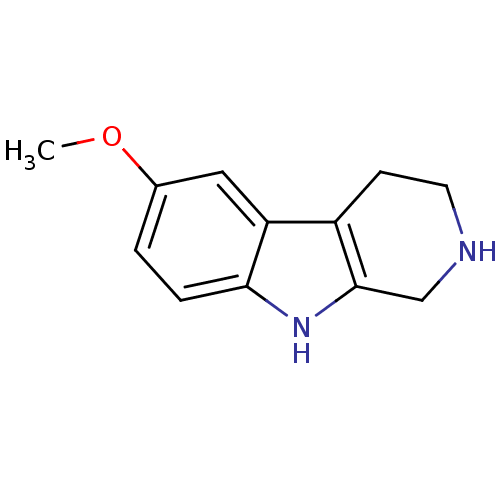 (6-Methoxy-2,3,4,9-tetrahydro-1H-beta-carboline | 6...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.27E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Naresuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine iodide as substrate measured every 5 sec for 2 mins by Ellman's method | Bioorg Med Chem Lett 22: 2885-8 (2012) Article DOI: 10.1016/j.bmcl.2012.02.057 BindingDB Entry DOI: 10.7270/Q2251K5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||