

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50271142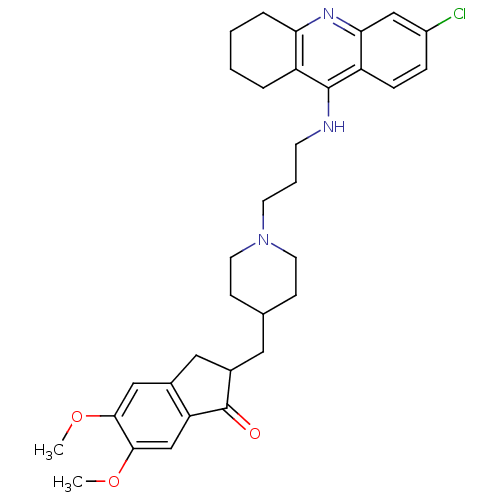 (6-Chloro-9-[(3-{4-[(5,6-Dimethoxy-1-oxoindan-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Binding affinity determined against ETA receptor in porcine aortic smooth muscle membrane | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by U... | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50277655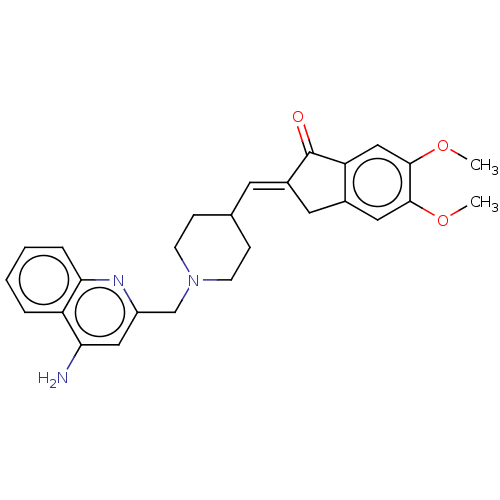 (CHEMBL4166300) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by U... | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 m... | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by U... | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50277652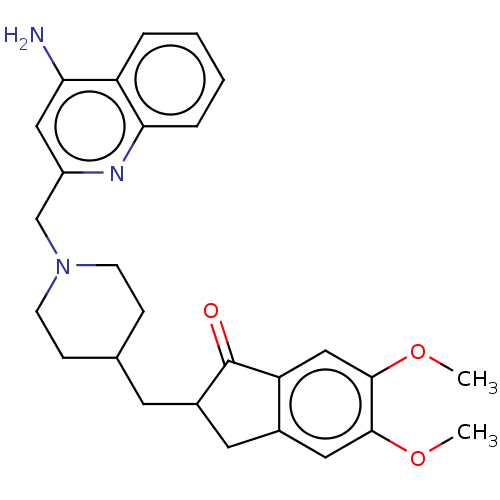 (CHEMBL4174269) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Binding affinity determined against ETA receptor in porcine aortic smooth muscle membrane | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50277655 (CHEMBL4166300) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Binding affinity determined against ETA receptor in porcine aortic smooth muscle membrane | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50277650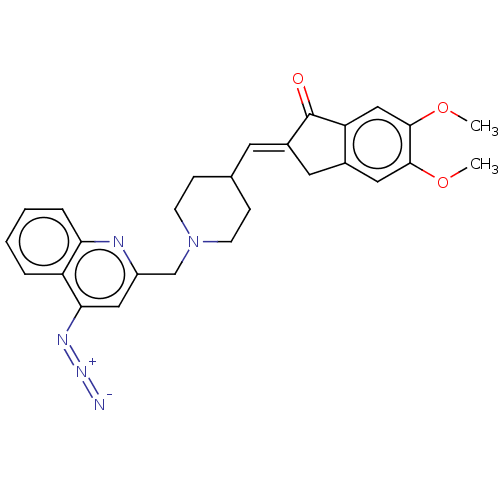 (CHEMBL4172587) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by U... | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50277651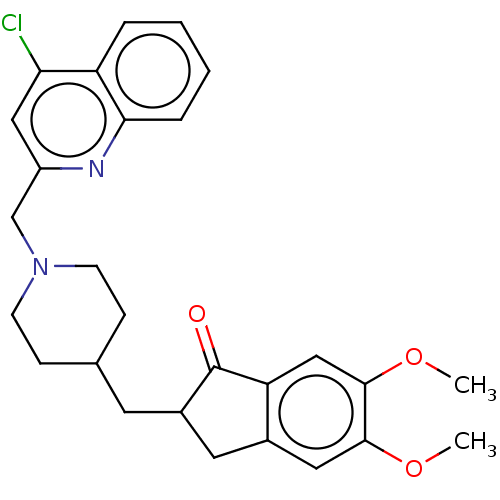 (CHEMBL4174514) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 m... | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50277652 (CHEMBL4174269) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 m... | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 m... | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50277664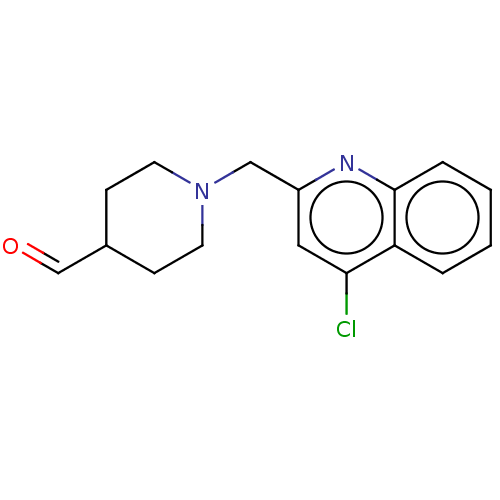 (CHEMBL4164010) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 m... | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50277654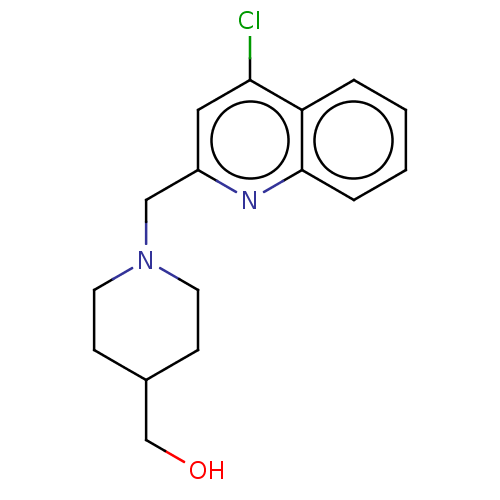 (CHEMBL4163705) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 m... | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50277656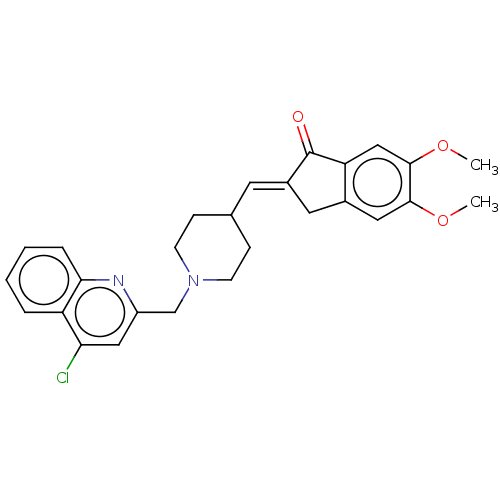 (CHEMBL4164347) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by U... | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50277664 (CHEMBL4164010) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by U... | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50277656 (CHEMBL4164347) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 m... | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50277654 (CHEMBL4163705) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Binding affinity determined against ETA receptor in porcine aortic smooth muscle membrane | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50277653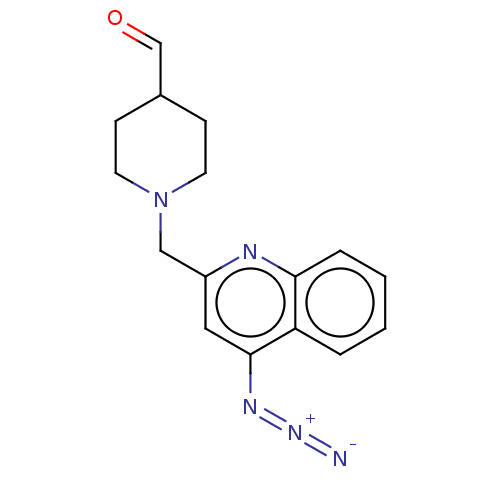 (CHEMBL4176598) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Binding affinity determined against ETA receptor in porcine aortic smooth muscle membrane | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50277653 (CHEMBL4176598) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Binding affinity determined against ETA receptor in porcine aortic smooth muscle membrane | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50277650 (CHEMBL4172587) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Binding affinity determined against ETA receptor in porcine aortic smooth muscle membrane | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50277651 (CHEMBL4174514) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Binding affinity determined against ETA receptor in porcine aortic smooth muscle membrane | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||