

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50422411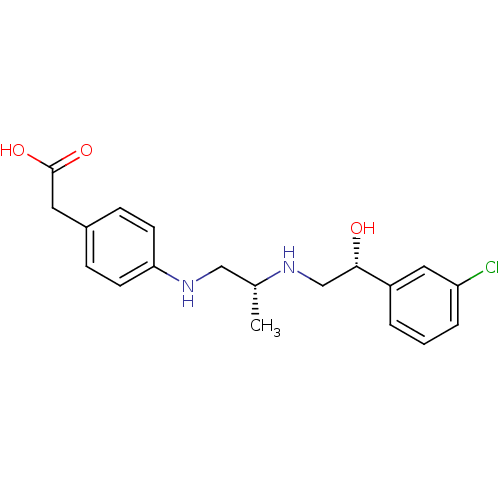 (CHEMBL154419) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 794 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Receptor binding assay(Beta-2 adrenergic receptor) carried out with membranes prepared from human recombinant Sf9 cells expressing the cloned human r... | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50422411 (CHEMBL154419) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Receptor binding assay(Beta-1 adrenergic receptor) carried out with membranes prepared from human recombinant Sf9 cells expressing the cloned human r... | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50422410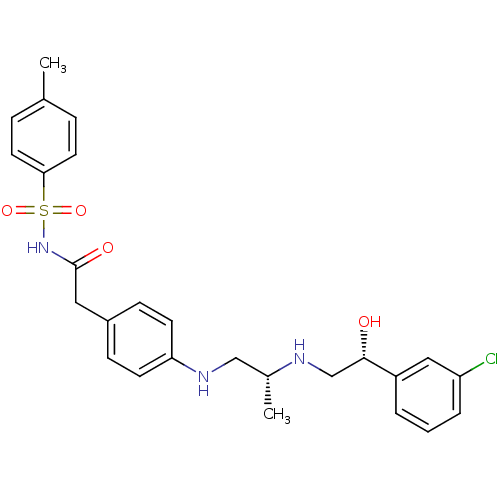 (CHEMBL348501) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Receptor binding assay(Beta-1 adrenergic receptor) carried out with membranes prepared from human recombinant Sf9 cells expressing the cloned human r... | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50422409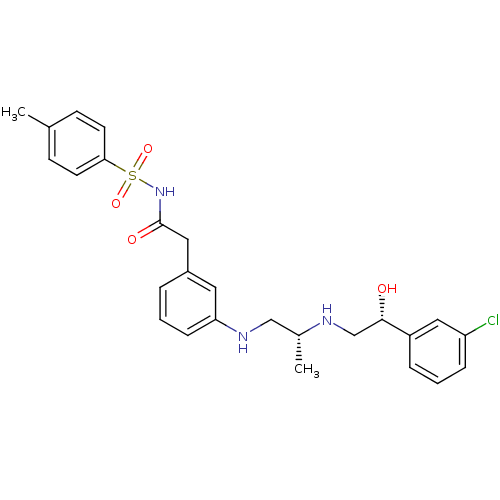 (CHEMBL347582) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Receptor binding assay(Beta-1 adrenergic receptor) carried out with membranes prepared from human recombinant Sf9 cells expressing the cloned human r... | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50422410 (CHEMBL348501) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Receptor binding assay(Beta-2 adrenergic receptor) carried out with membranes prepared from human recombinant Sf9 cells expressing the cloned human r... | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50422409 (CHEMBL347582) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Receptor binding assay(Beta-2 adrenergic receptor) carried out with membranes prepared from human recombinant Sf9 cells expressing the cloned human r... | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50027815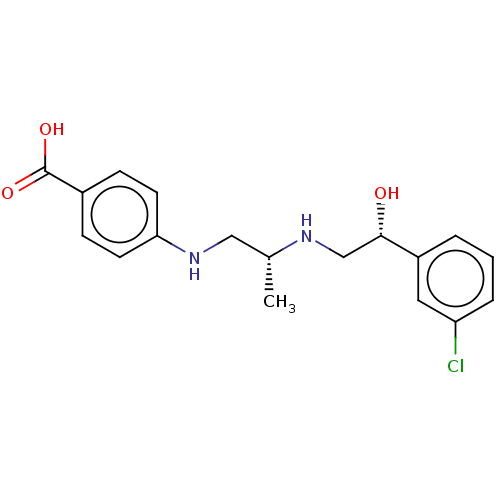 (CHEMBL155092) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 631 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-1 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50422410 (CHEMBL348501) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 79 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-2 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50027812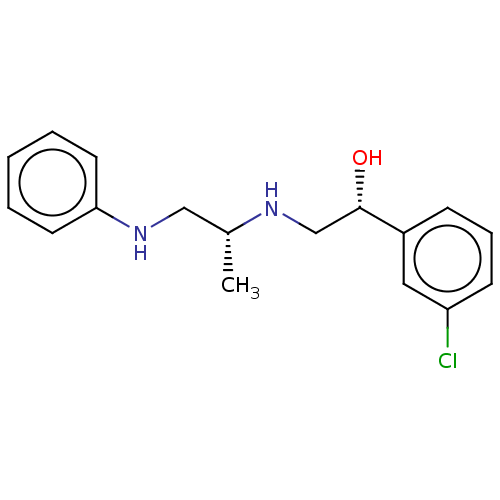 (CHEMBL155072) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-2 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50422411 (CHEMBL154419) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 79 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-1 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50027810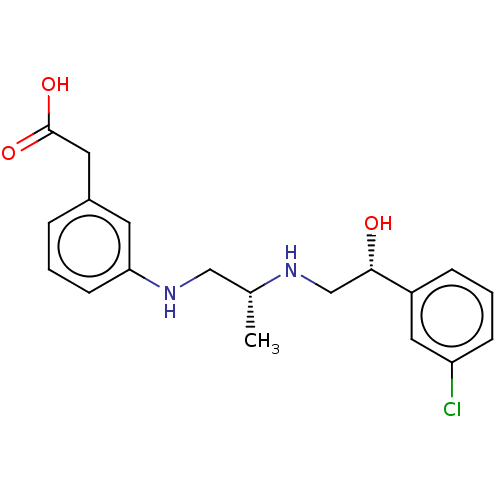 (CHEMBL154036) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 316 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-3 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50027810 (CHEMBL154036) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-1 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50027823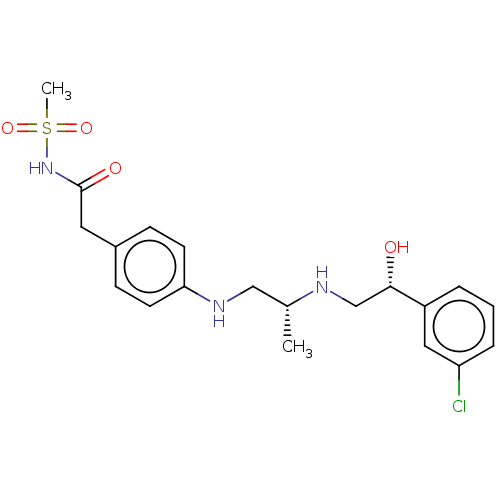 (CHEMBL157210) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-2 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50027821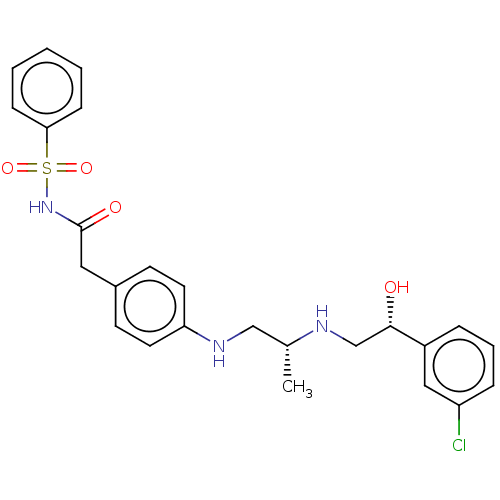 (CHEMBL347616) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-2 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50027822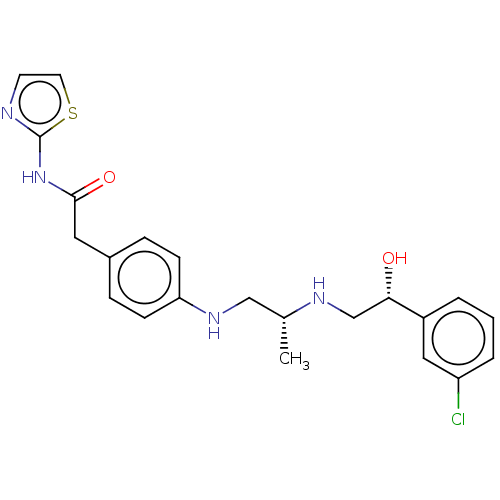 (CHEMBL154150) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-3 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50027816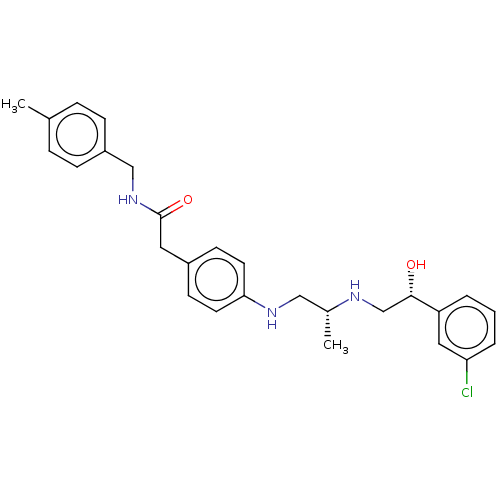 (CHEMBL154370) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 63 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-3 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50027818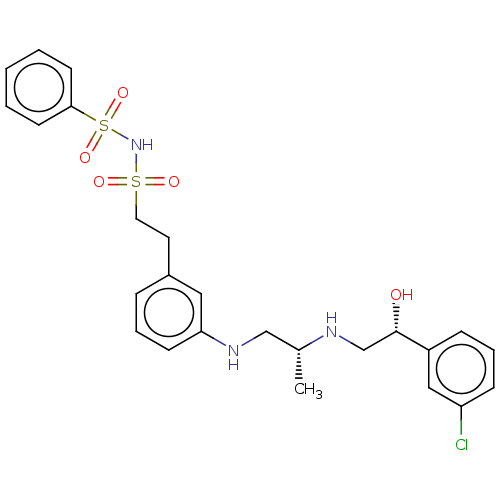 (CHEMBL347400) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | n/a | <794 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Activity against human beta 1 adrenergic receptor (AR), expressed in CHO cells. | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50027816 (CHEMBL154370) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-1 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50027811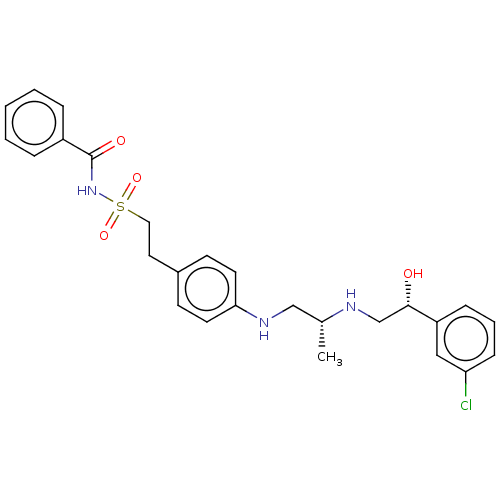 (CHEMBL422876) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 794 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Concentration that causes 50% inhibition of human beta 1 adrenergic receptor (AR), expressed in CHO cells. | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50027822 (CHEMBL154150) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-2 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50027819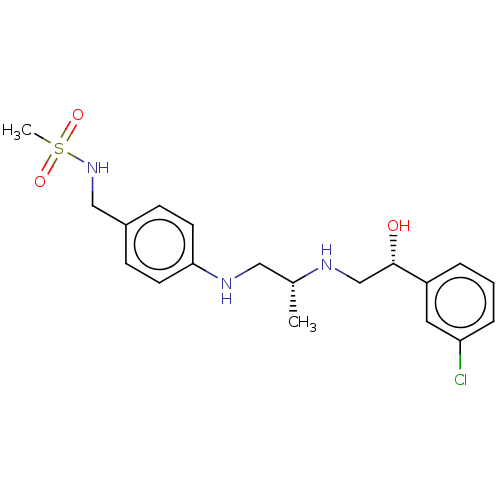 (CHEMBL153916) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-1 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50002598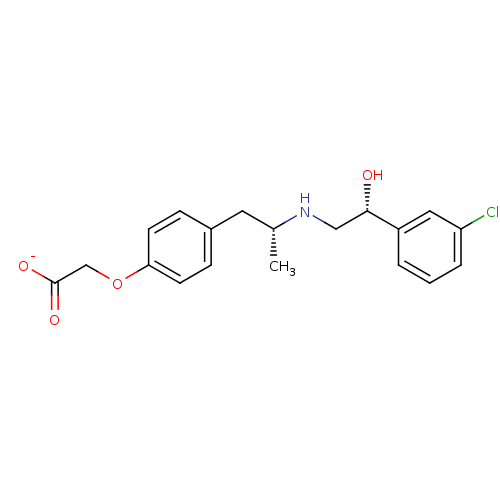 (CHEMBL152535) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | <794 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-1 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50422409 (CHEMBL347582) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-3 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50027818 (CHEMBL347400) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Activity against human beta 2 adrenergic receptor (AR), expressed in CHO cells | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50027823 (CHEMBL157210) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-3 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50027814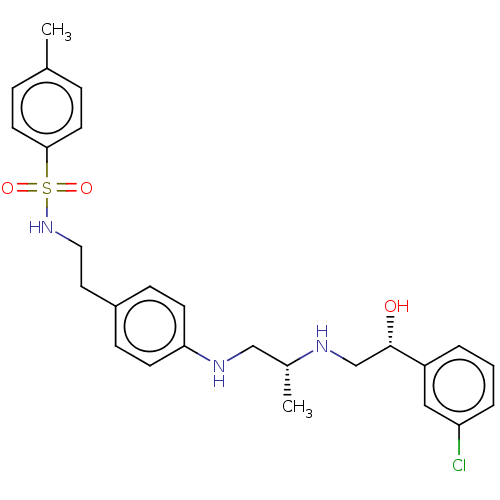 (CHEMBL348269) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-3 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50027821 (CHEMBL347616) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-3 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM25392 (4-[1-hydroxy-2-(isopropylamino)ethyl]pyrocatechol;...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Activity against human beta 1 adrenergic receptor (AR), expressed in CHO cells. | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50422410 (CHEMBL348501) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 631 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-1 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50027813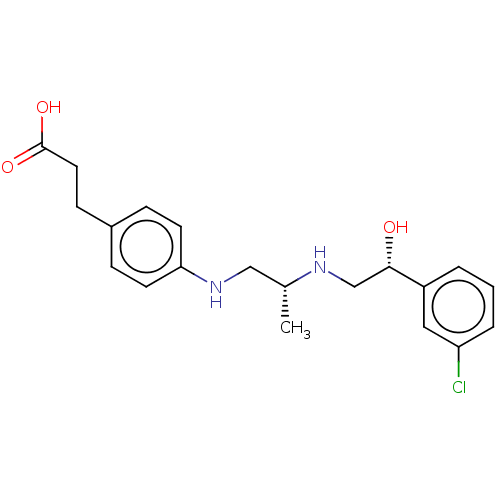 (CHEMBL153558) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 251 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-1 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50027815 (CHEMBL155092) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-2 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50027817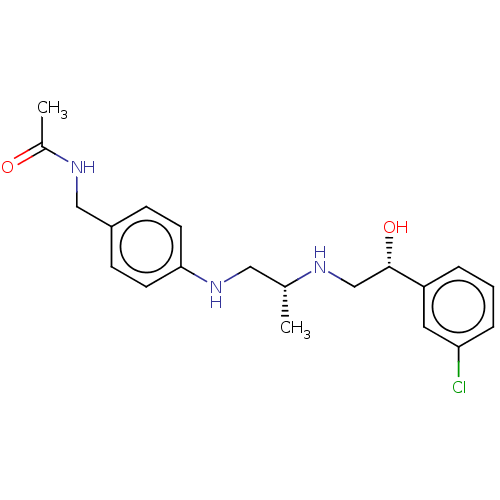 (CHEMBL345304) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-3 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50027817 (CHEMBL345304) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-1 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50027814 (CHEMBL348269) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-2 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50027810 (CHEMBL154036) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-2 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50027823 (CHEMBL157210) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-1 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50027809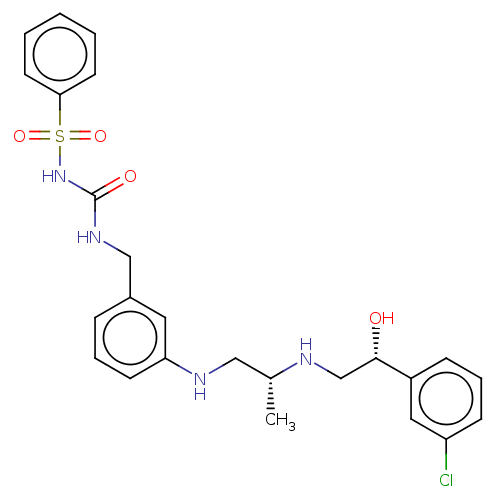 (CHEMBL154906) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 251 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Concentration that causes 50% inhibition of human beta 2 adrenergic receptor (AR), expressed in CHO cells. | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50422409 (CHEMBL347582) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Concentration that causes 50% inhibition of human beta 2 adrenergic receptor (AR), expressed in CHO cells. | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50027813 (CHEMBL153558) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-3 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50422410 (CHEMBL348501) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-3 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50027821 (CHEMBL347616) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 794 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-1 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50027812 (CHEMBL155072) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-3 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50027812 (CHEMBL155072) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-1 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50027809 (CHEMBL154906) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-3 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50422411 (CHEMBL154419) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-2 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50002598 (CHEMBL152535) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-3 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50027819 (CHEMBL153916) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-3 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50027816 (CHEMBL154370) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-2 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50027822 (CHEMBL154150) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Ability to cause cAMP accumulation in CHO cells expressing human beta-1 AR expressed as the negative logarithm of the molar drug concentration | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM25392 (4-[1-hydroxy-2-(isopropylamino)ethyl]pyrocatechol;...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Activity against human beta 3 adrenergic receptor (AR), expressed in CHO cells. | J Med Chem 45: 567-83 (2002) BindingDB Entry DOI: 10.7270/Q2W0976P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 63 total ) | Next | Last >> |